Page 495 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 495
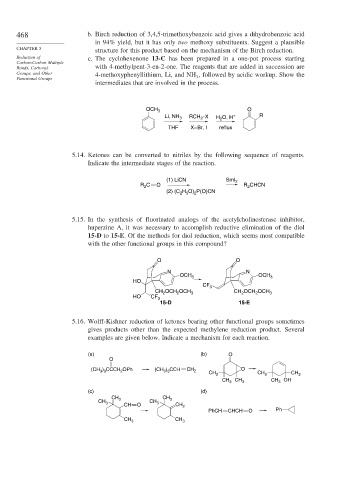
468 b. Birch reduction of 3,4,5-trimethoxybenzoic acid gives a dihydrobenzoic acid
in 94% yield, but it has only two methoxy substituents. Suggest a plausible
CHAPTER 5
structure for this product based on the mechanism of the Birch reduction.
Reduction of c. The cyclohexenone 13-C has been prepared in a one-pot process starting
Carbon-Carbon Multiple
Bonds, Carbonyl with 4-methylpent-3-en-2-one. The reagents that are added in succession are
Groups, and Other 4-methoxyphenyllithium, Li, and NH , followed by acidic workup. Show the
Functional Groups 3
intermediates that are involved in the process.
O
OCH 3
Li, NH 3 RCH –X H O, H + R
2
2
THF X=Br, I reflux
5.14. Ketones can be converted to nitriles by the following sequence of reagents.
Indicate the intermediate stages of the reaction.
(1) LiCN SmI 2
R C O R CHCN
2
2
(2) (C H O) P(O)CN
2 5
2
5.15. In the synthesis of fluorinated analogs of the acetylcholinesterase inhibitor,
huperzine A, it was necessary to accomplish reductive elimination of the diol
15-D to 15-E. Of the methods for diol reduction, which seems most compatible
with the other functional groups in this compound?
O O
N N
OCH 3 OCH 3
HO
CF 3
CH OCH OCH 3 CH 2 OCH OCH 3
2
2
2
HO CF 3
15-D 15-E
5.16. Wolff-Kishner reduction of ketones bearing other functional groups sometimes
gives products other than the expected methylene reduction product. Several
examples are given below. Indicate a mechanism for each reaction.
(a) (b) O
O
(CH ) CCCH OPh (CH ) CCH CH 2 O
3 3
2
3 3
CH 3 CH 3 CH 3
CH 3 CH 3 CH 3 OH
(c) (d)
CH
CH 3 3
CH 3 CH 3
CH O CH 2
PhCH CHCH O Ph
CH 3 CH 3