Page 497 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 497
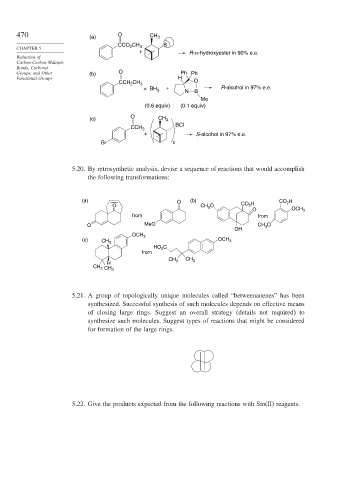
470 (a) O CH 3
CCO CH B
CHAPTER 5 2 3
+ R-α-hydroxyester in 90% e.e.
Reduction of
Carbon-Carbon Multiple
Bonds, Carbonyl
Groups, and Other (b) O Ph Ph
Functional Groups H
CCH CH 3 O
2
+ R-alcohol in 97% e.e.
+ BH 3
N B
Me
(0.6 equiv) (0.1 equiv)
(c) O CH 3
BCl
CCH 3
+ S-alcohol in 97% e.e.
Br 2
5.20. By retrosynthetic analysis, devise a sequence of reactions that would accomplish
the following transformations:
(a) O (b) CO H
2
O CH O CO H
2
3
O OCH 3
from from
O MeO CH O
3
OH
OCH 3
(c) CH 3 OCH 3
HO C
2
from
CH CH
H 3 3
CH 3 CH
3
5.21. A group of topologically unique molecules called “betweenanenes” has been
synthesized. Successful synthesis of such molecules depends on effective means
of closing large rings. Suggest an overall strategy (details not required) to
synthesize such molecules. Suggest types of reactions that might be considered
for formation of the large rings.
5.22. Give the products expected from the following reactions with Sm(II) reagents.