Page 502 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 502
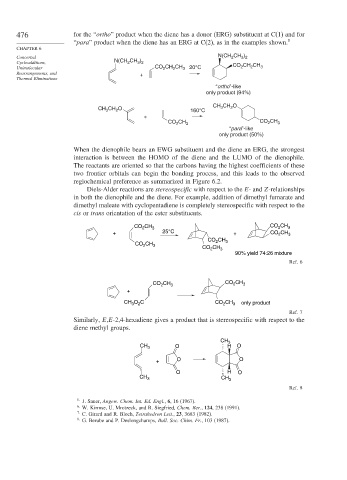
476 for the “ortho” product when the diene has a donor (ERG) substituent at C(1) and for
“para” product when the diene has an ERG at C(2), as in the examples shown. 5
CHAPTER 6
Concerted N(CH 2 CH 3 ) 2
2
3 2
Cycloadditions, N(CH CH ) CO CH CH
Unimolecular CO CH CH 3 20°C 2 2 3
2
2
Rearrangements, and +
Thermal Eliminations
“ortho”-like
only product (94%)
CH 3 CH 2 O
CH CH O 160°C
2
3
+
CO CH 3 CO 2 CH 3
2
“para”-like
only product (50%)
When the dienophile bears an EWG substituent and the diene an ERG, the strongest
interaction is between the HOMO of the diene and the LUMO of the dienophile.
The reactants are oriented so that the carbons having the highest coefficients of these
two frontier orbitals can begin the bonding process, and this leads to the observed
regiochemical preference as summarized in Figure 6.2.
Diels-Alder reactions are stereospecific with respect to the E- and Z-relationships
in both the dienophile and the diene. For example, addition of dimethyl fumarate and
dimethyl maleate with cyclopentadiene is completely stereospecific with respect to the
cis or trans orientation of the ester substituents.
CO CH
CO 2 CH 3 2 3
+ 25°C + CO CH 3
2
CO CH
CO CH 3 2 3
2
CO CH 3
2
90% yield 74:26 mixture
Ref. 6
CO CH 3 CO CH 3
2
2
+
CH O C CO CH 3 only product
2
3
2
Ref. 7
Similarly, E,E-2,4-hexadiene gives a product that is stereospecific with respect to the
diene methyl groups.
CH 3
CH 3 O H O
+ O O
O H O
CH 3 CH 3
Ref. 8
5
J. Sauer, Angew. Chem. Int. Ed. Engl., 6, 16 (1967).
6
W. Kirmse, U. Mrotzeck, and R. Siegfried, Chem. Ber., 124, 238 (1991).
7 C. Girard and R. Bloch, Tetrahedron Lett., 23, 3683 (1982).
8
G. Berube and P. Deslongchamps, Bull. Soc. Chim. Fr., 103 (1987).