Page 507 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 507
groups. 2-t-Butyl-1,3-butadiene is 27 times more reactive than butadiene. The t-butyl 481
substituent favors the s-cis conformation because of steric repulsions in the s-trans
conformation. SECTION 6.1
Diels-Alder Reactions
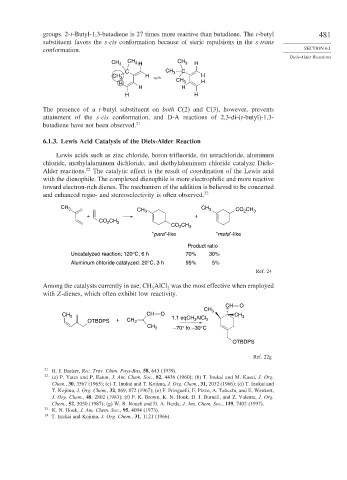
CH 3 CH 3 H CH 3 H
C CH 3 C
H H
CH 3
CH 3 H
H
H H
H H
The presence of a t-butyl substituent on both C(2) and C(3), however, prevents
attainment of the s-cis conformation, and D-A reactions of 2,3-di-(t-butyl)-1,3-
butadiene have not been observed. 21
6.1.3. Lewis Acid Catalysis of the Diels-Alder Reaction
Lewis acids such as zinc chloride, boron trifluoride, tin tetrachloride, aluminum
chloride, methylaluminum dichloride, and diethylaluminum chloride catalyze Diels-
Alder reactions. 22 The catalytic effect is the result of coordination of the Lewis acid
with the dienophile. The complexed dienophile is more electrophilic and more reactive
toward electron-rich dienes. The mechanism of the addition is believed to be concerted
and enhanced regio- and stereoselectivity is often observed. 23
CH 3 CH 3 CH 3 CO 2 CH 3
+ +
CO CH 3
2
CO CH 3
2
“para”-like “meta”-like
Product ratio
Uncatalyzed reaction: 120°C, 6 h 70% 30%
Aluminum chloride catalyzed: 20°C, 3 h 95% 5%
Ref. 24
Among the catalysts currently in use, CH AlCl was the most effective when employed
2
3
with Z-dienes, which often exhibit low reactivity.
CH O
CH 3
CH 3 CH O 1.1 eqCH AlCl CH 3
OTBDPS + CH 2 3 2
CH 3 –70° to –30°C
OTBDPS
Ref. 22g
21 H. J. Backer, Rec. Trav. Chim. Pays-Bas, 58, 643 (1939).
22
(a) P. Yates and P. Eaton, J. Am. Chem. Soc., 82, 4436 (1960); (b) T. Inukai and M. Kasai, J. Org.
Chem., 30, 3567 (1965); (c) T. Inukai and T. Kojima, J. Org. Chem., 31, 2032 (1966); (d) T. Inukai and
T. Kojima, J. Org. Chem., 32, 869, 872 (1967); (e) F. Fringuelli, F. Pizzo, A. Taticchi, and E. Wenkert,
J. Org. Chem., 48, 2802 (1983); (f) F. K. Brown, K. N. Houk, D. J. Burnell, and Z. Valenta, J. Org.
Chem., 52, 3050 (1987); (g) W. R. Roush and D. A. Barda, J. Am. Chem. Soc., 119, 7402 (1997).
23 K. N. Houk, J. Am. Chem. Soc., 95, 4094 (1973).
24
T. Inukai and Kojima, J. Org. Chem., 31, 1121 (1966).