Page 509 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 509
483
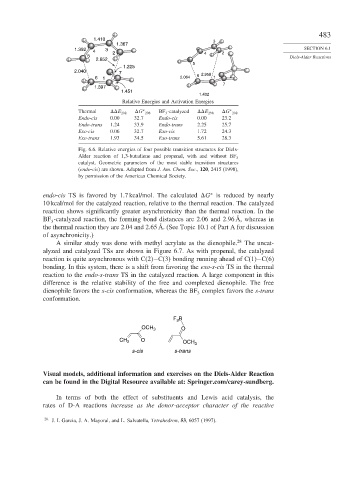
1.410 3
1.367
1.392 3 SECTION 6.1
4 2 2
5 4 Diels-Alder Reactions
2.652
5
1.225
2.040 7 2.958
6 1 2.064 6 1
7
1.397
1.451
1.402
Relative Energies and Activation Energies
Thermal
E 298
G 298 BF 3 -catalyzed
E 298
G 298
∗
∗
Endo-cis 0.00 32 7 Endo-cis 0.00 23.2
Endo-trans 1.24 33 9 Endo-trans 2.25 25.7
Exo-cis 0.06 32 7 Exo-cis 1.72 24.3
Exo-trans 1.93 34 5 Exo-trans 5.61 28.3
Fig. 6.6. Relative energies of four possible transition structures for Diels-
Alder reaction of 1,3-butadiene and propenal, with and without BF 3
catalyst. Geometric parameters of the most stable transition structures
(endo-cis) are shown. Adapted from J. Am. Chem. Soc., 120, 2415 (1998),
by permission of the American Chemical Society.
endo-cis TS is favored by 1.7 kcal/mol. The calculated
G is reduced by nearly
∗
10 kcal/mol for the catalyzed reaction, relative to the thermal reaction. The catalyzed
reaction shows significantly greater asynchronicity than the thermal reaction. In the
BF -catalyzed reaction, the forming bond distances are 2.06 and 2.96 Å, whereas in
3
the thermal reaction they are 2.04 and 2.65 Å. (See Topic 10.1 of Part A for discussion
of asynchronicity.)
A similar study was done with methyl acrylate as the dienophile. 28 The uncat-
alyzed and catalyzed TSs are shown in Figure 6.7. As with propenal, the catalyzed
reaction is quite asynchronous with C(2)−C(3) bonding running ahead of C(1)−C(6)
bonding. In this system, there is a shift from favoring the exo-s-cis TS in the thermal
reaction to the endo-s-trans TS in the catalyzed reaction. A large component in this
difference is the relative stability of the free and complexed dienophile. The free
dienophile favors the s-cis conformation, whereas the BF complex favors the s-trans
3
conformation.
F B
3
OCH 3 O
CH 2 O OCH 3
s-cis s-trans
Visual models, additional information and exercises on the Diels-Alder Reaction
can be found in the Digital Resource available at: Springer.com/carey-sundberg.
In terms of both the effect of substituents and Lewis acid catalysis, the
rates of D-A reactions increase as the donor-acceptor character of the reactive
28
J. I. Garcia, J. A. Mayoral, and L. Salvatella, Tetrahedron, 53, 6057 (1997).