Page 510 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 510
484
1.356 1.403 1.397
1.365
CHAPTER 6
1.394 1.389
2.661
Concerted
2.531
Cycloadditions, 1.248 1.908 1.986
Unimolecular 1.243
Rearrangements, and 1.316 1.307 1.400
Thermal Eliminations 1.412 1.413 1.427
TS endo s-cis
TS endo s-trans
1.400
1.361
1.397
1.365
1.391
1.388
1.942 2.504
1.411
1.400 2.003
1.418 1.429
1.247 1.239 1.309
1.311
TS exo s-cis
TS exo s-trans
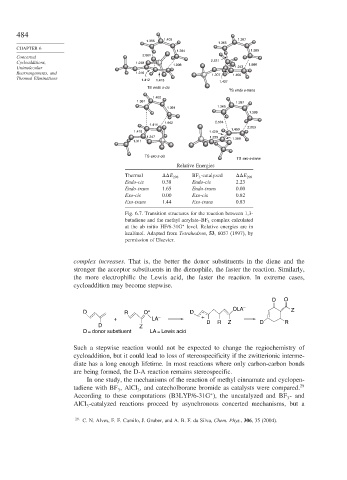
Relative Energies
Thermal
E 298 BF 3 -catalyzed
E 298
Endo-cis 0.38 Endo-cis 2.23
Endo-trans 1.65 Endo-trans 0.00
Exo-cis 0.00 Exo-cis 0.82
Exo-trans 1.44 Exo-trans 0.83
Fig. 6.7. Transition structures for the reaction between 1,3-
butadiene and the methyl acrylate–BF 3 complex calculated
at the ab initio HF/6-31G level. Relative energies are in
∗
kcal/mol. Adapted from Tetrahedron, 53, 6057 (1997), by
permission of Elsevier.
complex increases. That is, the better the donor substituents in the diene and the
stronger the acceptor substituents in the dienophile, the faster the reaction. Similarly,
the more electrophilic the Lewis acid, the faster the reaction. In extreme cases,
cycloaddition may become stepwise.
D O
OLA –
D R O + D Z
+ LA – +
D Z D R Z D R
D = donor substiuent LA = Lewis acid
Such a stepwise reaction would not be expected to change the regiochemistry of
cycloaddition, but it could lead to loss of stereospecificity if the zwitterionic interme-
diate has a long enough lifetime. In most reactions where only carbon-carbon bonds
are being formed, the D-A reaction remains stereospecific.
In one study, the mechanisms of the reaction of methyl cinnamate and cyclopen-
tadiene with BF , AlCl , and catecholborane bromide as catalysts were compared. 29
3
3
∗
According to these computations (B3LYP/6-31G ), the uncatalyzed and BF - and
3
AlCl -catalyzed reactions proceed by asynchronous concerted mechanisms, but a
3
29
C. N. Alves, F. F. Camilo, J. Gruber, and A. B. F. da Silva, Chem. Phys., 306, 35 (2004).