Page 508 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 508
482
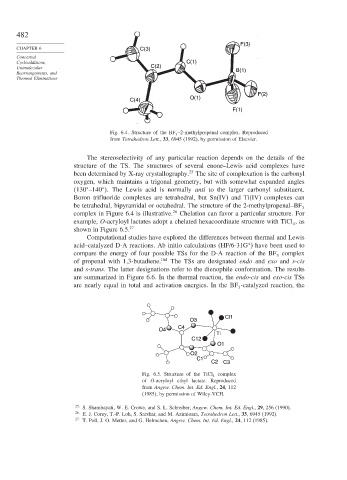
F(3)
CHAPTER 6 C(3)
Concerted
Cycloadditions, C(1)
Unimolecular C(2) B(1)
Rearrangements, and
Thermal Eliminations
F(2)
O(1)
C(4)
F(1)
Fig. 6.4. Structure of the BF 3 –2-methylpropenal complex. Reproduced
from Tetrahedron Lett., 33, 6945 (1992), by permission of Elsevier.
The stereoselectivity of any particular reaction depends on the details of the
structure of the TS. The structures of several enone–Lewis acid complexes have
been determined by X-ray crystallography. 25 The site of complexation is the carbonyl
oxygen, which maintains a trigonal geometry, but with somewhat expanded angles
130 –140 . The Lewis acid is normally anti to the larger carbonyl substituent.
Boron trifluoride complexes are tetrahedral, but Sn(IV) and Ti(IV) complexes can
be tetrahedral, bipyramidal or octahedral. The structure of the 2-methylpropenal–BF
3
complex in Figure 6.4 is illustrative. 26 Chelation can favor a particular structure. For
example, O-acryloyl lactates adopt a chelated hexacoordinate structure with TiCl ,as
4
shown in Figure 6.5. 27
Computational studies have explored the differences between thermal and Lewis
acid–catalyzed D-A reactions. Ab initio calculations (HF/6-31G ) have been used to
∗
compare the energy of four possible TSs for the D-A reaction of the BF complex
3
of propenal with 1,3-butadiene. 16d The TSs are designated endo and exo and s-cis
and s-trans. The latter designations refer to the dienophile conformation. The results
are summarized in Figure 6.6. In the thermal reaction, the endo-cis and exo-cis TSs
are nearly equal in total and activation energies. In the BF -catalyzed reaction, the
3
Cl1
O3
C4
O4
Ti
C12
O1
O2
C1
C2 C3
Fig. 6.5. Structure of the TiCl 4 complex
of O-acryloyl ethyl lactate. Reproduced
from Angew. Chem. Int. Ed. Engl., 24, 112
(1985), by permission of Wiley-VCH.
25 S. Shambayati, W. E. Crowe, and S. L. Schreiber, Angew. Chem. Int. Ed. Engl., 29, 256 (1990).
26 E. J. Corey, T.-P. Loh, S. Sarshar, and M. Azimioara, Tetrahedron Lett., 33, 6945 (1992).
27
T. Poll, J. O. Metter, and G. Helmchen, Angew. Chem. Int. Ed. Engl., 24, 112 (1985).