Page 513 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 513
CH 3 CH 3 487
O O
O N
N H HO C SECTION 6.1
2
N O
N
CH 3 H NH O Diels-Alder Reactions
1 N H O N
O O H
+ O N
CO H O O
2
N O H
O
2
O
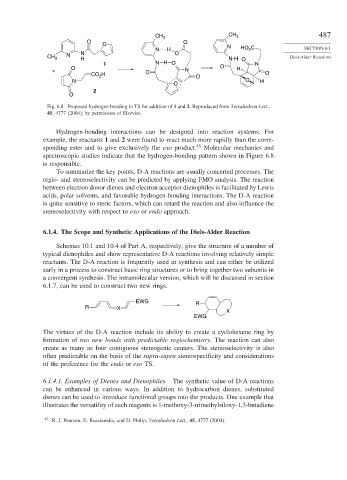
Fig. 6.8. Proposed hydrogen bonding in TS for addition of 1 and 2. Reproduced from Tetrahedron Lett.,
45, 4777 (2004), by permission of Elsevier.
Hydrogen-bonding interactions can be designed into reaction systems. For
example, the reactants 1 and 2 were found to react much more rapidly than the corre-
sponding ester and to give exclusively the exo product. 45 Molecular mechanics and
spectroscopic studies indicate that the hydrogen-bonding pattern shown in Figure 6.8
is responsible.
To summarize the key points, D-A reactions are usually concerted processes. The
regio- and stereoselectivity can be predicted by applying FMO analysis. The reaction
between electron donor dienes and electron acceptor dienophiles is facilitated by Lewis
acids, polar solvents, and favorable hydrogen-bonding interactions. The D-A reaction
is quite sensitive to steric factors, which can retard the reaction and also influence the
stereoselectivity with respect to exo or endo approach.
6.1.4. The Scope and Synthetic Applications of the Diels-Alder Reaction
Schemes 10.1 and 10.4 of Part A, respectively, give the structure of a number of
typical dienophiles and show representative D-A reactions involving relatively simple
reactants. The D-A reaction is frequently used in synthesis and can either be utilized
early in a process to construct basic ring structures or to bring together two subunits in
a convergent synthesis. The intramolecular version, which will be discussed in section
6.1.7, can be used to construct two new rings.
EWG R
R X
X
EWG
The virtues of the D-A reaction include its ability to create a cyclohexene ring by
formation of two new bonds with predictable regiochemistry. The reaction can also
create as many as four contiguous stereogenic centers. The stereoselectivity is also
often predictable on the basis of the supra-supra stereospecificity and considerations
of the preference for the endo or exo TS.
6.1.4.1. Examples of Dienes and Dienophiles. The synthetic value of D-A reactions
can be enhanced in various ways. In addition to hydrocarbon dienes, substituted
dienes can be used to introduce functional groups into the products. One example that
illustrates the versatility of such reagents is 1-methoxy-3-trimethylsiloxy-1,3-butadiene
45
R. J. Pearson, E. Kassianidis, and D. Philip, Tetrahedron Lett., 45, 4777 (2004).