Page 518 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 518
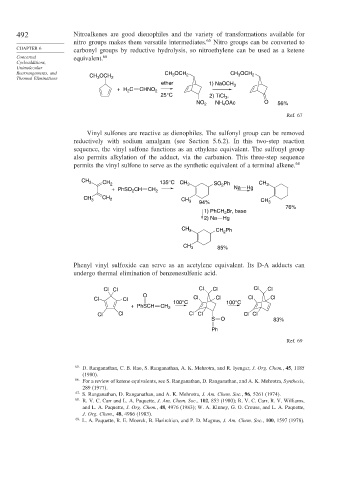
492 Nitroalkenes are good dienophiles and the variety of transformations available for
nitro groups makes them versatile intermediates. 65 Nitro groups can be converted to
CHAPTER 6
carbonyl groups by reductive hydrolysis, so nitroethylene can be used as a ketene
Concerted equivalent. 66
Cycloadditions,
Unimolecular
Rearrangements, and CH OCH CH OCH 2 CH 3 OCH 2
3
Thermal Eliminations 3 2
ether 1) NaOCH 3
+H C CHNO 2
2
25°C 2) TiCl ,
3
NO 2 NH OAc O 56%
4
Ref. 67
Vinyl sulfones are reactive as dienophiles. The sulfonyl group can be removed
reductively with sodium amalgam (see Section 5.6.2). In this two-step reaction
sequence, the vinyl sulfone functions as an ethylene equivalent. The sulfonyl group
also permits alkylation of the adduct, via the carbanion. This three-step sequence
permits the vinyl sulfone to serve as the synthetic equivalent of a terminal alkene. 68
CH 3 CH 2 135°C CH 3 SO Ph CH 3
+ PhSO CH CH 2 2 Na Hg
2
CH 3 CH 2 CH 3 94% CH 3
76%
Br, base
1) PhCH 2
2) Na Hg
CH 3 CH Ph
2
CH 3 85%
Phenyl vinyl sulfoxide can serve as an acetylene equivalent. Its D-A adducts can
undergo thermal elimination of benzenesulfenic acid.
Cl Cl Cl Cl Cl Cl
O
Cl Cl Cl Cl Cl Cl
100°C 100°C
+ PhSCH CH 2
Cl Cl Cl Cl Cl Cl
S O 83%
Ph
Ref. 69
65 D. Ranganathan, C. B. Rao, S. Ranganathan, A. K. Mehrotra, and R. Iyengar, J. Org. Chem., 45, 1185
(1980).
66
For a review of ketene equivalents, see S. Ranganathan, D. Ranganathan, and A. K. Mehrotra, Synthesis,
289 (1977).
67 S. Ranganathan, D. Ranganathan, and A. K. Mehrotra, J. Am. Chem. Soc., 96, 5261 (1974).
68 R. V. C. Carr and L. A. Paquette, J. Am. Chem. Soc., 102, 853 (1980); R. V. C. Carr, R. V. Williams,
and L. A. Paquette, J. Org. Chem., 48, 4976 (1983); W. A. Kinney, G. O. Crouse, and L. A. Paquette,
J. Org. Chem., 48, 4986 (1983).
69
L. A. Paquette, R. E. Moerck, B. Harirchian, and P. D. Magnus, J. Am. Chem. Soc., 100, 1597 (1978).