Page 522 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 522
496 2 illustrates the use of high pressure to accelerate reaction. This reaction gives
only an endo product, since both the electronic effect of the formyl group and the
CHAPTER 6
steric effect of the sulfonamido group favor this orientation. The reaction in Entry
Concerted 3 involves a typical diene and dienophiles. The reaction is completely regiospe-
Cycloadditions,
Unimolecular cific in the direction expected [donor alkyl groups at C(1) and C(3) of the diene
Rearrangements, and unit] and is also completely endo selective. The facial selectivity with respect to
Thermal Eliminations
the diene, however, is only 3.3:1. Entry 4 is an example of the use of nitroethene
as an ethene equivalent. The nitro group was removed by reduction with Bu SnH.
3
The reaction in Entry 5 involves a diene unit activated by a 2-siloxy substituent. On
exposure to acid, this provides the product as a ketone. The reaction is evidently
completely regio- and stereoselective. Entry 6 involves a doubly activated diene.
The aromatic ring is formed by extrusion of isobutylene from a bicyclic interme-
diate. Entry 7 involves the ring opening of a benzocyclobutene to a quinodimethane.
In this case, aromatization occurs as the result of the loss of two methoxy groups.
Owing to their advantages in terms of the lower temperature required and the
higher regio- and stereoselectivity, Lewis acid–catalyzed D-A reactions are often
preferable to the corresponding thermal version. Scheme 6.2 gives some examples of
D-A reactions catalyzed by Lewis acids. Entries 1 and 2 are cases with substituent
groups on the reacting bonds. Systems of this type are often relatively unreactive in
thermal D-A reactions. The reaction in Entry 2 is an inverse electron demand case, and
the catalyst activates the diene rather than the dienophile. Entry 3 involves a relatively
highly substituted diene. The reaction was used to create a structure corresponding
to the A-ring of the antitumor substance taxol. Entries 4, 5, and 6 involve dienes
that have donor substituents that impart regioselectivity. The products of each of the
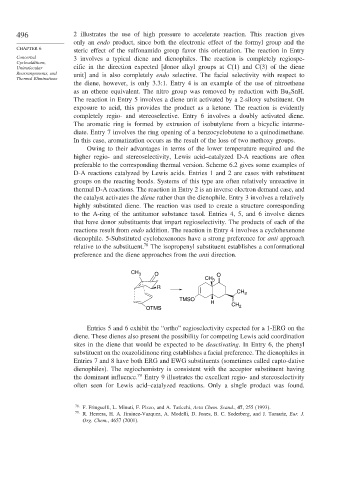
reactions result from endo addition. The reaction in Entry 4 involves a cyclohexenone
dienophile. 5-Substituted cyclohexenones have a strong preference for anti approach
relative to the substituent. 78 The isopropenyl substituent establishes a conformational
preference and the diene approaches from the anti direction.
CH 3 O O
CH 3
– R
CH 3
TMSO
H CH
OTMS 2
Entries 5 and 6 exhibit the “ortho” regioselectivity expected for a 1-ERG on the
diene. These dienes also present the possibility for competing Lewis acid coordination
sites in the diene that would be expected to be deactivating. In Entry 6, the phenyl
substituent on the oxazolidinone ring establishes a facial preference. The dienophiles in
Entries 7 and 8 have both ERG and EWG substituents (sometimes called capto-dative
dienophiles). The regiochemistry is consistent with the acceptor substituent having
the dominant influence. 79 Entry 9 illustrates the excellent regio- and stereoselectivity
often seen for Lewis acid–catalyzed reactions. Only a single product was found.
78 F. Fringuelli, L. Minuti, F. Pizzo, and A. Taticchi, Acta Chem. Scand., 47, 255 (1993).
79
R. Herrera, H. A. Jiminez-Vazquez, A. Modelli, D. Jones, B. C. Soderberg, and J. Tamariz, Eur. J.
Org. Chem., 4657 (2001).