Page 525 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 525
499
O Al
CH 3
SECTION 6.1
O Diels-Alder Reactions
O OCH 3
CH 3 CH 3
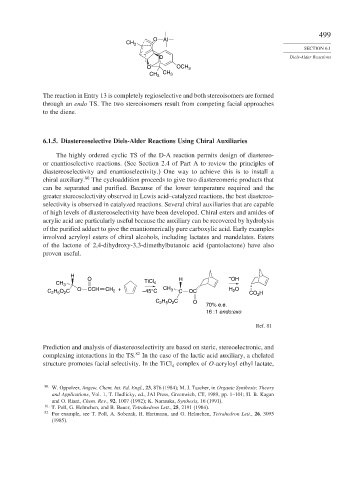
The reaction in Entry 13 is completely regioselective and both stereoisomers are formed
through an endo TS. The two stereoisomers result from competing facial approaches
to the diene.
6.1.5. Diastereoselective Diels-Alder Reactions Using Chiral Auxiliaries
The highly ordered cyclic TS of the D-A reaction permits design of diastereo-
or enantioselective reactions. (See Section 2.4 of Part A to review the principles of
diastereoselectivity and enantioselectivity.) One way to achieve this is to install a
80
chiral auxiliary. The cycloaddition proceeds to give two diastereomeric products that
can be separated and purified. Because of the lower temperature required and the
greater stereoselectivity observed in Lewis acid–catalyzed reactions, the best diastereo-
selectivity is observed in catalyzed reactions. Several chiral auxiliaries that are capable
of high levels of diastereoselectivity have been developed. Chiral esters and amides of
acrylic acid are particularly useful because the auxiliary can be recovered by hydrolysis
of the purified adduct to give the enantiomerically pure carboxylic acid. Early examples
involved acryloyl esters of chiral alcohols, including lactates and mandelates. Esters
of the lactone of 2,4-dihydroxy-3,3-dimethylbutanoic acid (pantolactone) have also
proven useful.
H
O H – OH
CH 3 TiCl 4
C H O C O CCH CH + –45°C CH 3 C OC H O CO H
2
2
2
2 5
2
C H O C O
2 5
2
70% e.e.
16 :1 endo:exo
Ref. 81
Prediction and analysis of diastereoselectivity are based on steric, stereoelectronic, and
82
complexing interactions in the TS. In the case of the lactic acid auxiliary, a chelated
structure promotes facial selectivity. In the TiCl complex of O-acryloyl ethyl lactate,
4
80
W. Oppolzer, Angew. Chem. Int. Ed. Engl., 23, 876 (1984); M. J. Tascher, in Organic Synthesis: Theory
and Applications, Vol. 1, T. Hudlicky, ed., JAI Press, Greenwich, CT, 1989, pp. 1–101; H. B. Kagan
and O. Riant, Chem. Rev., 92, 1007 (1992); K. Narasaka, Synthesis, 16 (1991).
81 T. Poll, G. Helmchen, and B. Bauer, Tetrahedron Lett., 25, 2191 (1984).
82
For example, see T. Poll, A. Sobczak, H. Hartmann, and G. Helmchen, Tetrahedron Lett., 26, 3095
(1985).