Page 529 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 529
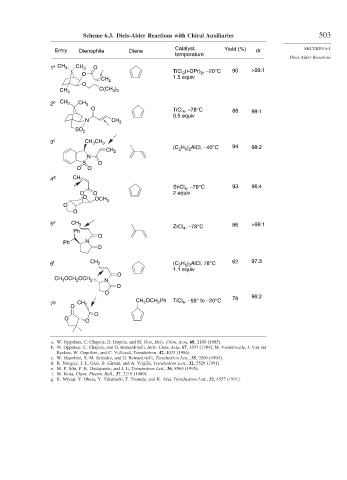
Scheme 6.3. Diels-Alder Reactions with Chiral Auxiliaries 503
Catalyst, Yield (%) SECTION 6.1
Entry Dienophile Diene dr
temperature
Diels-Alder Reactions
1 a CH 3 CH 3 O (i-OPr) , –20°C 90 >99:1
O TiCl 2 2
1.5 equiv
CH 2
O
CH 3 C(CH )
3 3
2 b CH 3 CH 3
O TiCl , –78°C 88 99:1
4
0.5 equiv
N CH 3
SO 2
3 c CH CH 3
3
(C H ) AlCl, –40°C 94 98:2
2 5 2
CH 2
N
S O
O O
4 d CH 2
SnCl , –78°C 93 96:4
4
O O 2 equiv
O OCH 3
O
O
5 e CH 3 86 >99:1
ZrCl 4 , –78°C
Ph
O
Ph N
O
6 f CH 3 (C H ) AlCl, 78°C 62 97:3
2 5 2
1.1 equiv
O
N
CH 3 OCH 2 OCH 2
O
O
79 96:2
CH OCH Ph TiCl , –55° to –20°C
7 g CH 2 2 2 4
O
O
O O
a. W. Oppolzer, C. Chapuis, D. Dupuis, and M. Guo, Helv. Chim. Acta, 68, 2100 (1985).
b. W. Oppolzer, C. Chapuis, and G. Bernardinelli, Helv. Chim. Acta, 67, 1397 (1984); M. Vanderwalle, J. Van der
Eycken, W. Oppolzer, and C. Vullioud, Tetrahedron, 42, 4035 (1986).
c. W. Oppolzer, B. M. Seletsky, and G. Bernardinelli, Tetrahedron Lett., 35, 3509 (1994).
d. R. Nougier, J.-L. Gras, B. Giraud, and A. Virgilli, Tetrahedron Lett., 32, 5529 (1991).
e. M. P. Sibi, P. K. Deshpande, and J. Ji, Tetrahedron Lett., 36, 8965 (1995).
f. M. Ikota, Chem. Pharm. Bull., 37, 2219 (1989).
g. K. Miyaji, Y. Ohara, Y. Takahashi, T. Tsuruda, and K. Arai, Tetrahedron Lett., 32, 4557 (1991).