Page 531 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 531
6.1.6. Enantioselective Catalysts for Diels-Alder Reactions 505
Enantioselectivity can also be achieved with chiral catalysts. The chiral oxazaboro- SECTION 6.1
lidinones introduced in Section 2.1.5.6 as enantioselective aldol addition catalysts have Diels-Alder Reactions
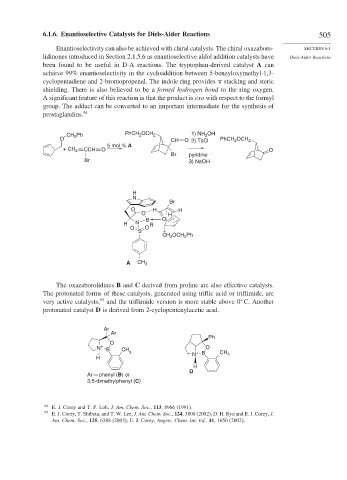
been found to be useful in D-A reactions. The tryptophan-derived catalyst A can
achieve 99% enantioselectivity in the cycloaddition between 5-benzyloxymethyl-1,3-
cyclopentadiene and 2-bromopropenal. The indole ring provides stacking and steric
shielding. There is also believed to be a formyl hydrogen bond to the ring oxygen.
A significant feature of this reaction is that the product is exo with respect to the formyl
group. The adduct can be converted to an important intermediate for the synthesis of
prostaglandins. 94
Ph PhCH OCH 2 1) NH OH
2
CH 2 2
O CH O 2) TsCl PhCH OCH 2
2
5 mol % A
CCH O
+ CH 2 O
Br pyridine
Br 3) NaOH
H
N
Br
O H H
O H
B O
H N R
O O
S
CH OCH Ph
2
2
A CH 3
The oxazaborolidines B and C derived from proline are also effective catalysts.
The protonated forms of these catalysts, generated using triflic acid or triflimide, are
very active catalysts, 95 and the triflimide version is more stable above 0 C. Another
protonated catalyst D is derived from 2-cyclopentenylacetic acid.
Ar
Ar
Ph
O
N + B CH O
3 N + B CH 3
H
H
D
Ar phenyl (B) or
3,5-dimethylphenyl (C)
94 E. J. Corey and T. P. Loh, J. Am. Chem. Soc., 113, 8966 (1991).
95
E. J. Corey, T. Shibata, and T. W. Lee, J. Am. Chem. Soc., 124, 3808 (2002); D. H. Ryu and E. J. Corey, J.
Am. Chem. Soc., 125, 6388 (2003); E. J. Corey, Angew. Chem. Int. Ed., 41, 1650 (2002).