Page 535 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 535
509
SECTION 6.1
C Diels-Alder Reactions
H
N
α-Si face
Cu
O
β N
α
α-Re face
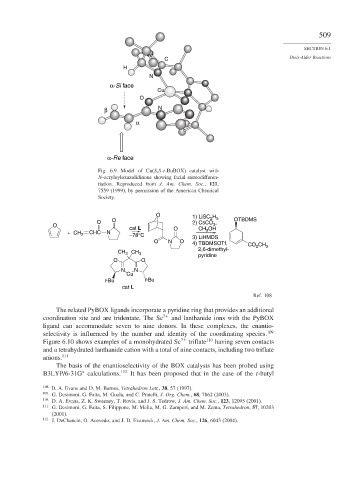
Fig. 6.9. Model of Cu(S,S-t-BuBOX) catalyst with
N-acryloyloxazolidinone showing facial stereodifferen-
tiation. Reproduced from J. Am. Chem. Soc., 121,
7559 (1999), by permission of the American Chemical
Society.
O 1) LiSC 2 5
H
O O 2) CsCO , OTBDMS
O 3
cat L O CH OH
CHC N 3
+ CH 2
–78°C 3) LiHMDS
O N O 4) TBDMSOTf, CO CH
2,6-dimethyl- 2 3
CH 3 CH 3 pyridine
O O
N
N –
Cu
t-Bu t-Bu
cat L
Ref. 108
The related PyBOX ligands incorporate a pyridine ring that provides an additional
coordination site and are tridentate. The Sc 3+ and lanthanide ions with the PyBOX
ligand can accommodate seven to nine donors. In these complexes, the enantio-
selectivity is influenced by the number and identity of the coordinating species. 109
Figure 6.10 shows examples of a monohydrated Sc 3+ triflate 110 having seven contacts
and a tetrahydrated lanthanide cation with a total of nine contacts, including two triflate
anions. 111
The basis of the enantioselectivity of the BOX catalysts has been probed using
∗
B3LYP/6-31G calculations. 112 It has been proposed that in the case of the t-butyl
108 D. A. Evans and D. M. Barnes, Tetrahedron Lett., 38, 57 (1997).
109 G. Desimoni, G. Faita, M. Guala, and C. Pratelli, J. Org. Chem., 68, 7862 (2003).
110
D. A. Evans, Z. K. Sweeney, T. Rovis, and J. S. Tedrow, J. Am. Chem. Soc., 123, 12095 (2001).
111 G. Desimoni, G. Faita, S. Filippone, M. Mella, M. G. Zampori, and M. Zema, Tetrahedron, 57, 10203
(2001).
112
J. DeChancie, O. Acevedo, and J. D. Evanseck, J. Am. Chem. Soc., 126, 6043 (2004).