Page 538 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 538
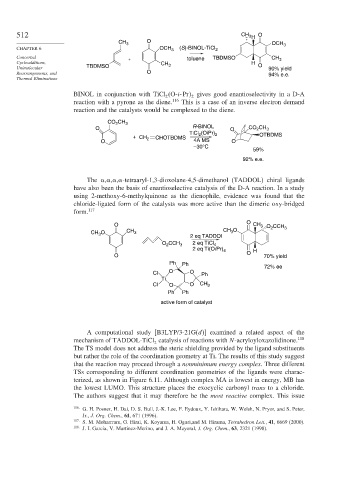
512 CH 3 H O
CH 3 O OCH 3
CHAPTER 6 OCH 3 (S)-BINOL-TiCl 2
Concerted + toluene TBDMSO CH 3
Cycloadditions, CH H
Unimolecular TBDMSO 3 O 90% yield
Rearrangements, and O 94% e.e.
Thermal Eliminations
BINOL in conjunction with TiCl O-i-Pr gives good enantioselectivity in a D-A
2 2
reaction with a pyrone as the diene. 116 This is a case of an inverse electron demand
reaction and the catalysts would be complexed to the diene.
CH
CO 2 3
O R-BINOL O CO 2 CH 3
(OiPr)
TiCl 2 2 OTBDMS
+ CH 2 CHOTBDMS
O 4A MS O
–30°C
59%
92% e.e.
The , , , -tetraaryl-1,3-dioxolane-4,5-dimethanol (TADDOL) chiral ligands
have also been the basis of enantioselective catalysis of the D-A reaction. In a study
using 2-methoxy-6-methylquinone as the dienophile, evidence was found that the
chloride-ligated form of the catalysts was more active than the dimeric oxy-bridged
form. 117
O
O CH 3 O CCH 3
2
CH O CH 3 CH 3 O
3
2 eq TADDOl
O CCH 3 2 eq TiCl 4
2
2 eq Ti(Oi Pr) 4 H
O O 70% yield
Ph Ph
72% ee
Cl O O Ph
Ti
Cl O O CH 3
Ph Ph
active form of catalyst
A computational study [B3LYP/3-21G(d)] examined a related aspect of the
mechanism of TADDOL-TiCl catalysis of reactions with N-acryloyloxazolidinone. 118
2
The TS model does not address the steric shielding provided by the ligand substituents
but rather the role of the coordination geometry at Ti. The results of this study suggest
that the reaction may proceed through a nonminimum energy complex. Three different
TSs corresponding to different coordination geometries of the ligands were charac-
terized, as shown in Figure 6.11. Although complex MA is lowest in energy, MB has
the lowest LUMO. This structure places the exocyclic carbonyl trans to a chloride.
The authors suggest that it may therefore be the most reactive complex. This issue
116
G. H. Posner, H. Dai, D. S. Bull, J.-K. Lee, F. Eydoux, Y. Ishihara, W. Welsh, N. Pryor, and S. Peter,
Jr., J. Org. Chem., 61, 671 (1996).
117 S. M. Moharrram, G. Hirai, K. Koyama, H. Oguri,and M. Hirama, Tetrahedron Lett., 41, 6669 (2000).
118
J. I. Garcia, V. Martinez-Merino, and J. A. Mayoral, J. Org. Chem., 63, 2321 (1998).