Page 536 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 536
510
CHAPTER 6
Concerted O 4
Cycloadditions,
Unimolecular
Rearrangements, and
Thermal Eliminations
Sc
O 1 O 2
O 3
C10 F1
C9
C11
F3
C19
O3
C8
F2
C12
S1 O4
C7
O2
O1 C2 C1
C5
C4 C3
N1
O6w i
C6
N2 O5w i
h
C14 C13 La1 N2 i
O5w
C15 O6w
C18
O4 i O2 i F2 i
C17 S1i
C16
C19 i
O3 i F3 i
F1 i
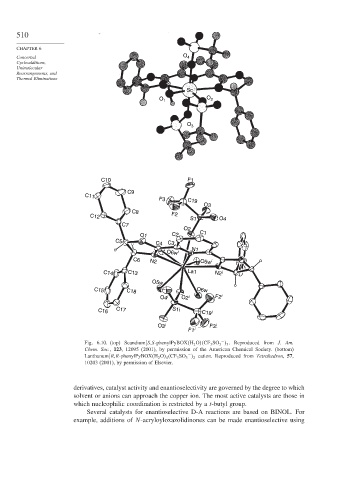
Fig. 6.10. (top) Scandium[S,S-phenylPyBOX H 2 O CF 3 SO 3 . Reproduced from J. Am.
−
3
Chem. Soc., 123, 12095 (2001), by permission of the American Chemical Society. (bottom)
−
Lanthanum[R,R-phenylPyBOX H 2 O 4 CF 3 SO 3 2 cation. Reproduced from Tetrahedron, 57,
10203 (2001), by permission of Elsevier.
derivatives, catalyst activity and enantioselectivity are governed by the degree to which
solvent or anions can approach the copper ion. The most active catalysts are those in
which nucleophilic coordination is restricted by a t-butyl group.
Several catalysts for enantioselective D-A reactions are based on BINOL. For
example, additions of N-acryloyloxazolidinones can be made enantioselective using