Page 532 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 532
506 -Unsaturated aldehydes react via TS E, whereas , -unsaturated ketones and esters
react via TS F.
CHAPTER 6
Concerted
Cycloadditions,
Unimolecular
Rearrangements, and
Thermal Eliminations CH 3 CH
O 3 O
N+ N+ H
H B H B
H R
O C O
E F
R
R
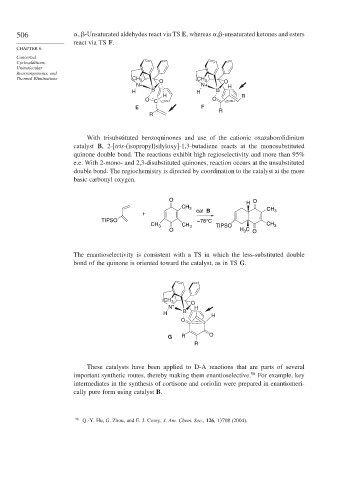
With trisubstituted benzoquinones and use of the cationic oxazaborolidinium
catalyst B, 2-[tris-(isopropyl)silyloxy]-1,3-butadiene reacts at the monosubstituted
quinone double bond. The reactions exhibit high regioselectivity and more than 95%
e.e. With 2-mono- and 2,3-disubstituted quinones, reaction occurs at the unsubstituted
double bond. The regiochemistry is directed by coordination to the catalyst at the more
basic carbonyl oxygen.
O O
H
CH 3 CH
+ cat B 3
TIPSO –78°C
CH 3 CH 3 TIPSO CH 3
O H C O
3
The enantioselectivity is consistent with a TS in which the less-substituted double
bond of the quinone is oriented toward the catalyst, as in TS G.
CH 3 O
N + H
H B H
O
G R O
R
These catalysts have been applied to D-A reactions that are parts of several
important synthetic routes, thereby making them enantioselective. 96 For example, key
intermediates in the synthesis of cortisone and coriolin were prepared in enantiomeri-
cally pure form using catalyst B.
96
Q.-Y. Hu, G. Zhou, and E. J. Corey, J. Am. Chem. Soc., 126, 13708 (2004).