Page 533 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 533
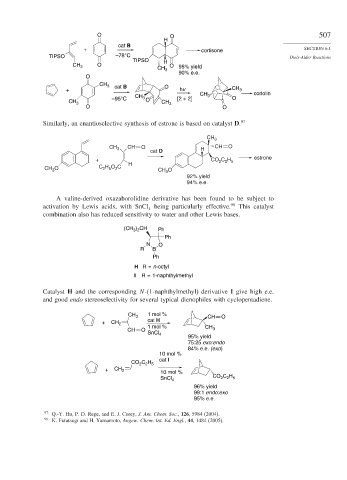
O O 507
H
cat B
+ cortisone SECTION 6.1
TIPSO –78°C Diels-Alder Reactions
TIPSO H
CH 3 O O 95% yield
CH 3
90% e.e.
O
CH 3 cat B O
+ hv CH 3
CH CH 3 coriolin
–95°C 3 O [2 + 2] O
CH 3 CH 3
O O
Similarly, an enantioselective synthesis of estrone is based on catalyst D. 97
CH 3
CH 3 CH O H CH O
cat D
+ CO C H estrone
2 2 5
H O C
CH O C 2 5 2 H CH 3 O
3
92% yield
94% e.e.
A valine-derived oxazaborolidine derivative has been found to be subject to
activation by Lewis acids, with SnCl being particularly effective. 98 This catalyst
4
combination also has reduced sensitivity to water and other Lewis bases.
(CH ) CH Ph
3 2
Ph
N O
R B
Ph
H R = n-octyl
I R = 1-naphthylmethyl
Catalyst H and the corresponding N-(1-naphthylmethyl) derivative I give high e.e.
and good endo stereoselectivity for several typical dienophiles with cyclopentadiene.
1 mol %
CH 3 CH O
+ CH 2 cat H
CH O 1 mol % CH 3
SnCl 4
95% yield
75:25 exo:endo
84% e.e. (exo)
10 mol %
cat I
CO C H
2 2 5
+ CH 2 10 mol %
2 2 5
SnCl 4 CO C H
96% yield
99:1 endo:exo
95% e.e.
97 Q.-Y. Hu, P. D. Rege, and E. J. Corey, J. Am. Chem. Soc., 126, 5984 (2004).
98
K. Futatsugi and H. Yamamoto, Angew. Chem. Int. Ed. Engl., 44, 1484 (2005).