Page 528 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 528
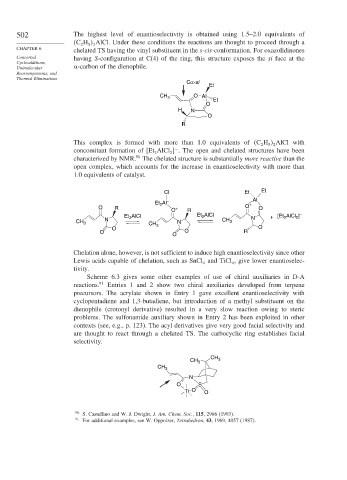
502 The highest level of enantioselectivity is obtained using 1.5–2.0 equivalents of
C H AlCl. Under these conditions the reactions are thought to proceed through a
2 5 2
CHAPTER 6
chelated TS having the vinyl substituent in the s-cis-conformation. For oxazolidinones
Concerted having S-configuration at C(4) of the ring, this structure exposes the si face at the
Cycloadditions,
Unimolecular -carbon of the dienophile.
Rearrangements, and
Thermal Eliminations
Cα-si
Et
O
CH 3 Al
Et
O
H N
O
R
This complex is formed with more than 1.0 equivalents of C H AlCl with
5 2
2
−
concomitant formation of Et AlCl . The open and chelated structures have been
2
2
90
characterized by NMR. The chelated structure is substantially more reactive than the
open complex, which accounts for the increase in enantioselectivity with more than
1.0 equivalents of catalyst.
Cl Et Et
Al
Et Al – +
2
O R O + R O O
Et 2 AlCl Et AlCl N + [Et 2 AlCl ] –
2
2
CH 3 N CH N CH 3
O 3 O
O O O R
Chelation alone, however, is not sufficient to induce high enantioselectivity since other
Lewis acids capable of chelation, such as SnCl and TiCl , give lower enantioselec-
4
4
tivity.
Scheme 6.3 gives some other examples of use of chiral auxiliaries in D-A
reactions. 91 Entries 1 and 2 show two chiral auxiliaries developed from terpene
precursors. The acrylate shown in Entry 1 gave excellent enantioselectivity with
cyclopentadiene and 1,3-butadiene, but introduction of a methyl substituent on the
dienophile (crotonyl derivative) resulted in a very slow reaction owing to steric
problems. The sulfonamide auxiliary shown in Entry 2 has been exploited in other
contexts (see, e.g., p. 123). The acyl derivatives give very good facial selectivity and
are thought to react through a chelated TS. The carbocyclic ring establishes facial
selectivity.
CH
CH 3 3
CH 3
N
O S
Ti O O
90 S. Castellino and W. J. Dwight, J. Am. Chem. Soc., 115, 2986 (1993).
91
For additional examples, see W. Oppolzer, Tetrahedron, 43, 1969, 4057 (1987).