Page 526 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 526
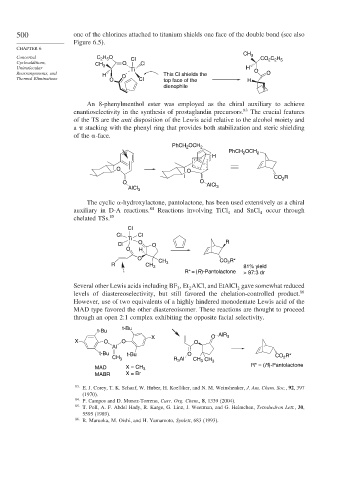
500 one of the chlorines attached to titanium shields one face of the double bond (see also
Figure 6.5).
CHAPTER 6
CH
Concerted C 2 5 Cl 3 CO C H
H O
Cycloadditions, O Cl 2 2 5
CH 3
Unimolecular Ti H
Rearrangements, and H This Cl shields the O O
Thermal Eliminations O O Cl top face of the H
dienophile
An 8-phenylmenthol ester was employed as the chiral auxiliary to achieve
enantioselectivity in the synthesis of prostaglandin precursors. 83 The crucial features
of the TS are the anti disposition of the Lewis acid relative to the alcohol moiety and
a stacking with the phenyl ring that provides both stabilization and steric shielding
of the -face.
PhCH 2 OCH 2
PhCH OCH 2
2
H
O O
CO R
2
O O
AlCl 3
AlCl 3
The cyclic -hydroxylactone, pantolactone, has been used extensively as a chiral
auxiliary in D-A reactions. 84 Reactions involving TiCl and SnCl occur through
4
4
chelated TSs. 85
Cl
Cl Cl
Ti
Cl O O R
O H
O CO R*
CH 3 2
R CH 3 81% yield
R* = (R)-Pantolactone > 97:3 dr
Several other Lewis acids including BF ,Et AlCl, and EtAlCl gave somewhat reduced
2
2
3
levels of diastereoselectivity, but still favored the chelation-controlled product. 86
However, use of two equivalents of a highly hindered monodentate Lewis acid of the
MAD type favored the other diastereoisomer. These reactions are thought to proceed
through an open 2:1 complex exhibiting the opposite facial selectivity.
t-Bu
t-Bu
X O AlR 3
X O O O
Al
t-Bu t-Bu O CO R*
CH 3 R Al CH CH 3 2
3
3
MAD X = CH 3 R* = (R)-Pantolactone
MABR X = Br
83
E. J. Corey, T. K. Schaaf, W. Huber, H. Koelliker, and N. M. Weinshenker, J. Am. Chem. Soc., 92, 397
(1970).
84 P. Campos and D. Munoz-Torreno, Curr. Org. Chem., 8, 1339 (2004).
85 T. Poll, A. F. Abdel Hady, R. Karge, G. Linz, J. Weetman, and G. Helmchen, Tetrahedron Lett., 30,
5595 (1989).
86
R. Maruoka, M. Oishi, and H. Yamamoto, Synlett, 683 (1993).