Page 524 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 524
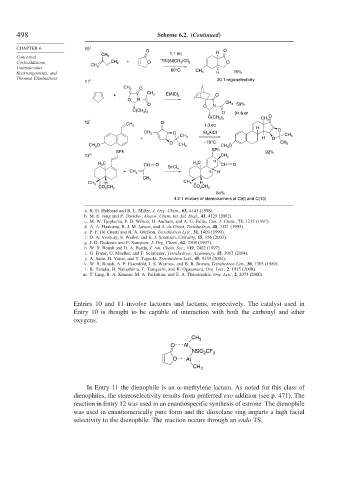
498 Scheme 6.2. (Continued)
CHAPTER 6 10 j
O O
1.1 eq H
Concerted CH 3
Cycloadditions, CH 2 + O TfN[Al(CH 3 )Cl] 2 O
CH 2
Unimolecular 60°C
Rearrangements, and CH 3 H 76%
Thermal Eliminations 20:1 regioselectivity
11 k
CH 2 O
+ CH 3 EtAlCl 2 O
O N
O O N CH 3 59%
C(CH 3 ) 3
O 94:6 dr
O
C(CH 3 ) 3 CH 3
12 l O
CH 2 1.3 eq
H
CH 3 O Et 2 AlCl O
+ CH 3 H O CH 3
O –78°C CH 3
CH 3 O CH 3 CH 3 O
SPh
SPh 92%
13 m CH 3
H 3 C CH O H 3 C 13 CH O
SnCl 4
+ CH 2 6 H
CH 3
H CH 3 H
CH 3
CO 2 CH 3 CO 2 CH 3
84%
4.2:1 mixture of stereoisomers at C(6) and C(13)
a. R. D. Hubbard and B. L. Miller, J. Org. Chem., 63, 4143 (1998).
b. M. E. Jung and P. Davidov, Angew. Chem. Int. Ed. Engl., 41, 4125 (2002).
c. M. W. Tjepkema, P. D. Wilson, H. Audrain, and A. G. Fallis, Can. J. Chem., 75, 1215 (1997).
d. A. A. Haaksma, B. J. M. Jansen, and A. de Groot, Tetrahedron, 48, 3121 (1992).
e. P. F. De Cusati and R. A. Olofson, Tetrahedron Lett., 31, 1409 (1990).
f. D. A. Vosburg, S. Weiler, and E. J. Sorensen, Chirality, 15, 156 (2003).
g. J. D. Dudones and P. Sampson, J. Org. Chem., 62, 7508 (1997).
h. W. R. Roush and D. A. Barda, J. Am. Chem. Soc., 119, 7402 (1997).
i. G. Frater, U. Mueller, and F. Schroeder, Tetrahedron: Asymmetry, 15, 3967 (2004).
j. A. Saito, H. Yanai, and T. Taguchi, Tetrahedron Lett., 45, 9439 (2004).
k. W. R. Roush, A. P. Essenfeld, J. S. Warmus, and B. B. Brown, Tetrahedron Lett., 30, 7305 (1989).
l. K. Tanaka, H. Nakashima, T. Taniguchi, and K. Ogasawara, Org. Lett., 2, 1915 (2000).
m. T. Ling, B. A. Kramer, M. A. Palladino, and E. A. Theodorakis, Org. Lett., 2, 2073 (2000).
Entries 10 and 11 involve lactones and lactams, respectively. The catalyst used in
Entry 10 is thought to be capable of interaction with both the carbonyl and ether
oxygens.
CH 3
O Al
CF
NSO 2 3
O Al
CH 3
In Entry 11 the dienophile is an -methylene lactam. As noted for this class of
dienophiles, the stereoselectivity results from preferred exo addition (see p. 471). The
reaction in Entry 12 was used in an enantiospecific synthesis of estrone. The dienophile
was used in enantiomerically pure form and the dioxolane ring imparts a high facial
selectivity to the dienophile. The reaction occurs through an endo TS.