Page 520 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 520
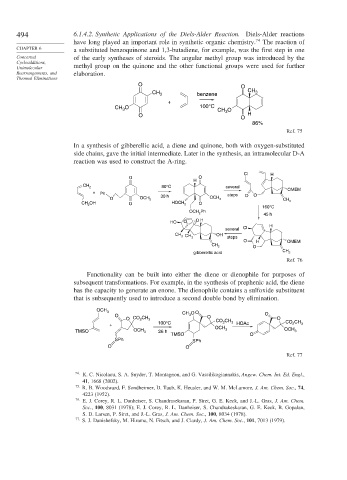
494 6.1.4.2. Synthetic Applications of the Diels-Alder Reaction. Diels-Alder reactions
have long played an important role in synthetic organic chemistry. 74 The reaction of
CHAPTER 6 a substituted benzoquinone and 1,3-butadiene, for example, was the first step in one
Concerted of the early syntheses of steroids. The angular methyl group was introduced by the
Cycloadditions,
Unimolecular methyl group on the quinone and the other functional groups were used for further
Rearrangements, and elaboration.
Thermal Eliminations
O
O
CH 3 benzene CH 3
+
CH O 100°C
3
CH 3 O
O O H
86%
Ref. 75
In a synthesis of gibberellic acid, a diene and quinone, both with oxygen-substituted
side chains, gave the initial intermediate. Later in the synthesis, an intramolecular D-A
reaction was used to construct the A-ring.
Cl H
O O
H
CH 80°C
2 several
OMEM
+ Ph steps O
O OCH 3 30 h OCH 3 O CH
CH OH O HOCH 2 O 2
2
160°C
OCH Ph
2 45 h
HO O O H
H
several Cl
OH
3 steps
CH CH 3
O H OMEM
CH 2
O
gibberellic acid CH 2
Ref. 76
Functionality can be built into either the diene or dienophile for purposes of
subsequent transformations. For example, in the synthesis of prephenic acid, the diene
has the capacity to generate an enone. The dienophile contains a sulfoxide substituent
that is subsequently used to introduce a second double bond by elimination.
OCH
3 CH O O O
O 3 O
O CO CH 3 CH O
2
2
+ 100°C CO 2 3 HOAc CO CH 3
TMSO OCH 3 26 h OCH 3 OCH 3
TMSO O
SPh SPh
O O
Ref. 77
74
K. C. Nicolaou, S. A. Snyder, T. Montagnon, and G. Vassilikogiannakis, Angew. Chem. Int. Ed. Engl.,
41, 1668 (2002).
75
R. B. Woodward, F. Sondheimer, D. Taub, K. Heusler, and W. M. McLamore, J. Am. Chem. Soc., 74,
4223 (1952).
76 E. J. Corey, R. L. Danheiser, S. Chandrasekaran, P. Siret, G. E. Keck, and J.-L. Gras, J. Am. Chem.
Soc., 100, 8031 (1978); E. J. Corey, R. L. Danheiser, S. Chandrakeskaran, G. E. Keck, B. Gopalan,
S. D. Larsen, P. Siret, and J.-L. Gras, J. Am. Chem. Soc., 100, 8034 (1978).
77
S. J. Danishefsky, M. Hirama, N. Fitsch, and J. Clardy, J. Am. Chem. Soc., 101, 7013 (1979).