Page 517 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 517
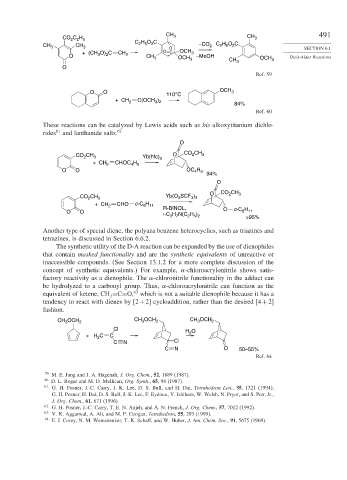
CH 491
CO C H 3 CH 3
2 2 5
2 5
2
CH 3 CH 3 C H O C O –CO 2 C H O C SECTION 6.1
2 5
2
+ (CH O) C CH 2 O C OCH 3
O 3 2 CH 3 OCH 3 –MeOH CH 3 OCH 3 Diels-Alder Reactions
O
Ref. 59
O O 110°C OCH 3
)
+ CH 2 C(OCH 3 2 84%
Ref. 60
These reactions can be catalyzed by Lewis acids such as bis-alkoxytitanium dichlo-
rides 61 and lanthanide salts. 62
O
2
CH O CO CH 3
CO 2 3 Yb(hfc) 3
+ CH 2 CHOC H
4 9
H
O O OC 4 9
94%
O
O CO CH 3
2
CO CH 3 Yb(O SCF )
3
3 3
2
+ CH 2 CHO c-C H
6 11
H
O O R-BINOL, O c-C 6 11
i-C H N(C H ) >95%
3 7
2 5 2
Another type of special diene, the polyaza benzene heterocyclics, such as triazines and
tetrazines, is discussed in Section 6.6.2.
The synthetic utility of the D-A reaction can be expanded by the use of dienophiles
that contain masked functionality and are the synthetic equivalents of unreactive or
inaccessible compounds. (See Section 13.1.2 for a more complete discussion of the
concept of synthetic equivalents.) For example, -chloroacrylonitrile shows satis-
factory reactivity as a dienophile. The -chloronitrile functionality in the adduct can
be hydrolyzed to a carbonyl group. Thus, -chloroacrylonitrile can function as the
63
equivalent of ketene, CH =C=O, which is not a suitable dienophile because it has a
2
tendency to react with dienes by 2+2 cycloaddition, rather than the desired 4+2
fashion.
OCH CH OCH OCH
CH 3 2 3 2 CH 3 2
Cl
H O
2
+H C C
2
C N Cl
C N O 50–55%
Ref. 64
59 M. E. Jung and J. A. Hagenah, J. Org. Chem., 52, 1889 (1987).
60
D. L. Boger and M. D. Mullican, Org. Synth., 65, 98 (1987).
61
G. H. Posner, J.-C. Carry, J. K. Lee, D. S. Bull, and H. Dai, Tetrahedron Lett., 35, 1321 (1994);
G. H. Posner, H. Dai, D. S. Bull, J.-K. Lee, F. Eydoux, Y. Ishihara, W. Welsh, N. Pryor, and S. Petr, Jr.,
J. Org. Chem., 61, 671 (1996).
62 G. H. Posner, J.-C. Carry, T. E. N. Anjeh, and A. N. French, J. Org. Chem., 57, 7012 (1992).
63 V. K. Aggarwal, A. Ali, and M. P. Coogan, Tetrahedron, 55, 293 (1999).
64
E. J. Corey, N. M. Weinshenker, T. K. Schaff, and W. Huber, J. Am. Chem. Soc., 91, 5675 (1969).