Page 516 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 516
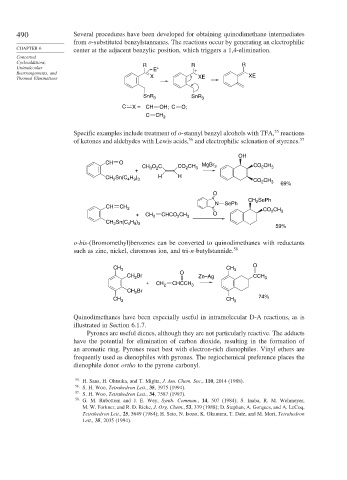
490 Several procedures have been developed for obtaining quinodimethane intermediates
from o-substituted benzylstannanes. The reactions occur by generating an electrophilic
CHAPTER 6 center at the adjacent benzylic position, which triggers a 1,4-elimination.
Concerted
Cycloadditions, R R R
Unimolecular E + +
Rearrangements, and XE
Thermal Eliminations X XE
SnR 3 SnR 3
C X = CH OH; C O;
C CH 2
55
Specific examples include treatment of o-stannyl benzyl alcohols with TFA, reactions
56
of ketones and aldehydes with Lewis acids, and electrophilic selenation of styrenes. 57
OH
CH O
CH O C CO CH 3 MgBr 2 CO CH 3
2
2
+ 3 2
Sn(C H ) H H
CH 2 4 9 3 CO CH 3 69%
2
O
CH SePh
2
N SePh
CH CH 2 CO CH
+ CH 2 CHCO CH 3 O 2 3
2
CH 2 Sn(C H )
4 9 3
59%
o-bis-(Bromomethyl)benzenes can be converted to quinodimethanes with reductants
such as zinc, nickel, chromous ion, and tri-n-butylstannide. 58
CH 3 CH 3 O
CH Br O Zn–Ag CCH 3
2
+ CH 2 CHCCH 3
CH 2 Br
CH 3 CH 3 74%
Quinodimethanes have been especially useful in intramolecular D-A reactions, as is
illustrated in Section 6.1.7.
Pyrones are useful dienes, although they are not particularly reactive. The adducts
have the potential for elimination of carbon dioxide, resulting in the formation of
an aromatic ring. Pyrones react best with electron-rich dienophiles. Vinyl ethers are
frequently used as dienophiles with pyrones. The regiochemical preference places the
dienophile donor ortho to the pyrone carbonyl.
55
H. Sans, H. Ohtsuka, and T. Migita, J. Am. Chem. Soc., 110, 2014 (1988).
56 S. H. Woo, Tetrahedron Lett., 35, 3975 (1994).
57 S. H. Woo, Tetrahedron Lett., 34, 7587 (1993).
58
G. M. Rubottom and J. E. Wey, Synth. Commun., 14, 507 (1984); S. Inaba, R. M. Wehmeyer,
M. W. Forkner, and R. D. Rieke, J. Org. Chem., 53, 339 (1988); D. Stephan, A. Gorques, and A. LeCoq,
Tetrahedron Lett., 25, 5649 (1984); H. Sato, N. Isono, K. Okamura, T. Date, and M. Mori, Tetrahedron
Lett., 35, 2035 (1994).