Page 511 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 511
stepwise mechanism is found with catecholborane bromide. Experimentally, this is the 485
only catalyst that is effective for this reaction. 30
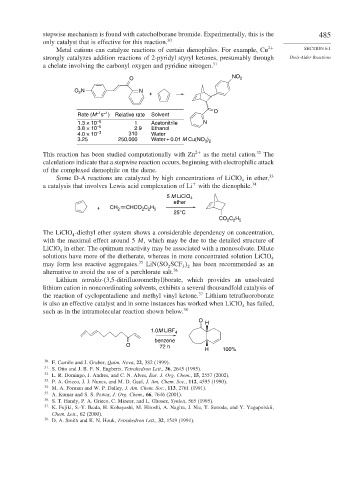
Metal cations can catalyze reactions of certain dienophiles. For example, Cu 2+ SECTION 6.1
strongly catalyzes addition reactions of 2-pyridyl styryl ketones, presumably through Diels-Alder Reactions
a chelate involving the carbonyl oxygen and pyridine nitrogen. 31
O NO 2
O N N +
2
–1 –1
Rate (M s ) Relative rate Solvent O
1.3 × 10 –5 1 Acetonitrile N
3.8 × 10 –5 2.9 Ethanol
4.0 × 10 –3 310 Water
3.25 250,000 Water + 0.01 M Cu(NO )
3 2
This reaction has been studied computationally with Zn 2+ as the metal cation. 32 The
calculations indicate that a stepwise reaction occurs, beginning with electrophilic attack
of the complexed dienophile on the diene.
Some D-A reactions are catalyzed by high concentrations of LiClO in ether, 33
4
+
a catalysis that involves Lewis acid complexation of Li with the dienophile. 34
5 M LiClO 4
ether
+ CH 2 CHCO C H
2 2 5
25°C
CO C H
2 2 5
The LiClO -diethyl ether system shows a considerable dependency on concentration,
4
with the maximal effect around 5 M, which may be due to the detailed structure of
LiClO in ether. The optimum reactivity may be associated with a monosolvate. Dilute
4
solutions have more of the dietherate, whereas in more concentrated solution LiClO
4
may form less reactive aggregates. 35 LiN SO SCF has been recommended as an
2 3 2
alternative to avoid the use of a perchlorate salt. 36
Lithium tetrakis-(3,5-ditrifluoromethyl)borate, which provides an unsolvated
lithium cation in noncoordinating solvents, exhibits a several thousandfold catalysis of
the reaction of cyclopentadiene and methyl vinyl ketone. 37 Lithium tetrafluoroborate
is also an effective catalyst and in some instances has worked when LiClO has failed,
4
such as in the intramolecular reaction shown below. 38
O
H
1.0M LiBF
4
benzene
O 72 h
H 100%
30
F. Camilo and J. Gruber, Quim. Nova, 22, 382 (1999).
31 S. Otto and J. B. F. N. Engberts, Tetrahedron Lett., 36, 2645 (1995).
32 L. R. Domingo, J. Andres, and C. N. Alves, Eur. J. Org. Chem., 15, 2557 (2002).
33
P. A. Grieco, J. J. Nunes, and M. D. Gaul, J. Am. Chem. Soc., 112, 4595 (1990).
34 M. A. Forman and W. P. Dailey, J. Am. Chem. Soc., 113, 2761 (1991).
35
A. Kumar and S. S. Pawar, J. Org. Chem., 66, 7646 (2001).
36
S. T. Handy, P. A. Grieco, C. Mineur, and L. Ghosez, Synlett, 565 (1995).
37 K. Fujiki, S.-Y. Ikeda, H. Kobayashi, M. Hiroshi, A. Nagira, J. Nie, T. Sonoda, and Y. Yagupolskii,
Chem. Lett., 62 (2000).
38
D. A. Smith and K. N. Houk, Tetrahedron Lett., 32, 1549 (1991).