Page 515 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 515
Unstable dienes can be generated in situ in the presence of a dienophile. Among 489
the most useful examples are the ortho-quinodimethanes. These compounds are exceed-
ingly reactive as dienes because the cycloaddition reestablishes a benzenoid ring and SECTION 6.1
results in aromatic stabilization. 51 Diels-Alder Reactions
CH 2 X X
+
CH 2
quinodimethane
There are several general routes to quinodimethanes. One is pyrolysis of benzocy-
clobutenes. 52
CH
heat 2
CH 2
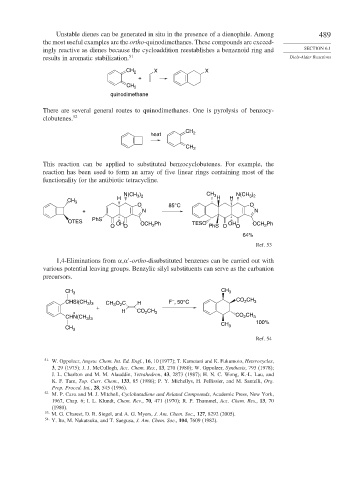
This reaction can be applied to substituted benzocyclobutenes. For example, the
reaction has been used to form an array of five linear rings containing most of the
functionality for the antibiotic tetracycline.
N(CH ) CH 3 N(CH )
3 2
3 2
CH 3 H H H
O 85°C O
+ N N
OTES PhS OH Ph TESO OH Ph
O O OCH 2 PhS O O OCH 2
64%
Ref. 53
1,4-Eliminations from , -ortho-disubstituted benzenes can be carried out with
various potential leaving groups. Benzylic silyl substituents can serve as the carbanion
precursors.
CH 3 CH 3
–
2
CHSi(CH ) CH O C H F , 50°C CO CH 3
3 3
+ 3 2
+ H CO 2 CH 3
CHN(CH ) CO CH 3
2
3 3
CH 100%
CH 3 3
Ref. 54
51
W. Oppolzer, Angew. Chem. Int. Ed. Engl., 16, 10 (1977); T. Kametani and K. Fukumoto, Heterocycles,
3, 29 (1975); J. J. McCullogh, Acc. Chem. Res., 13, 270 (1980); W. Oppolzer, Synthesis, 793 (1978);
J. L. Charlton and M. M. Alauddin, Tetrahedron, 43, 2873 (1987); H. N. C. Wong, K.-L. Lau, and
K. F. Tam, Top. Curr. Chem., 133, 85 (1986); P. Y. Michellys, H. Pellissier, and M. Santelli, Org.
Prep. Proced. Int., 28, 545 (1996).
52 M. P. Cava and M. J. Mitchell, Cyclobutadiene and Related Compounds, Academic Press, New York,
1967, Chap. 6; I. L. Klundt, Chem. Rev., 70, 471 (1970); R. P. Thummel, Acc. Chem. Res., 13,70
(1980).
53 M. G. Charest, D. R. Siegel, and A. G. Myers, J. Am. Chem. Soc., 127, 8292 (2005).
54
Y. Ito, M. Nakatsuka, and T. Saegusa, J. Am. Chem. Soc., 104, 7609 (1982).