Page 521 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 521
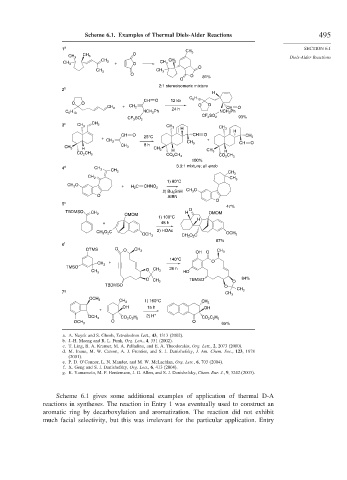
Scheme 6.1. Examples of Thermal Diels-Alder Reactions 495
1 a SECTION 6.1
CH 3
O
CH 3
CH 3 Diels-Alder Reactions
CH 2 CH 3
CH 3 + O CH 3
O
CH 3 CH 3
O O
O 81%
2:1 stereoisomeric mixture
2 b
H
CH O 12 kb C 6 H 13
O O O O
CH 2 + CH 2 24 h CH O
NCH 2 Ph NCH 2 Ph
C 6 H 13
CF 3 SO 2 93%
CF 3 SO 2
3 c CH 3 CH 2
CH 3
H CH 3
H
CH O CH O CH 3
+ 25°C +
CH 2
CH 3 CH O
8 h
CH 3
H CH 3 H CH 3 H
CH 3
CO 2 CH 3
CO 2 CH 3 CO 2 CH 3
100%
4 d CH 3 3.3:1 mixture; all endo
CH 3
CH 3
CH 2 CH 3
1) 80°C
CH 3 O + H 2 C CHNO 2
2) Bu 3 SnH CH 3 O
O AIBN
O
5 e
47%
O
TBDMSO H OMOM
OMOM
CH 2
1) 100°C
H
+ 48 h
CH 3 O 2 C 2) HOAc
OCH 3 CH 3 O 2 C OCH 3
87%
6 f
OTMS O O CH 3
OH O CH 3
140°C
+ O
CH 3
TMSO 36 h
O CH 3
CH 3 HO
TBMSO 84%
O
O CH 3
TBDMSO O
7 g CH 3
CH 3
OCH 3
CH 3 1) 160°C CH 3
OH 15 h OH
+
2) H +
OCH 3 CO 2 C 2 H 5 CO 2 C 2 H 5
O O
OCH 3 65%
a. A. Nayek and S. Ghosh, Tetrahedron Lett., 43, 1313 (2002).
b. J.-H. Maeng and R. L. Funk, Org. Lett., 4, 331 (2002).
c. T. Ling, B. A. Kramer, M. A. Palladino, and E. A. Theodorakis, Org. Lett., 2, 2073 (2000).
d. M. Inoue, M. W. Carson, A. J. Frontier, and S. J. Danishefsky, J. Am. Chem. Soc., 123, 1878
(2001).
e. P. D. O’Connor, L. N. Mander, and M. W. McLachlan, Org. Lett., 6, 703 (2004).
f. X. Geng and S. J. Danishefsky, Org. Lett., 6, 413 (2004).
g. K. Yamamoto, M. F. Hentemann, J. G. Allen, and S. J. Danishefsky, Chem. Eur. J., 9, 3242 (2003).
Scheme 6.1 gives some additional examples of application of thermal D-A
reactions in syntheses. The reaction in Entry 1 was eventually used to construct an
aromatic ring by decarboxylation and aromatization. The reaction did not exhibit
much facial selectivity, but this was irrelevant for the particular application. Entry