Page 537 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 537
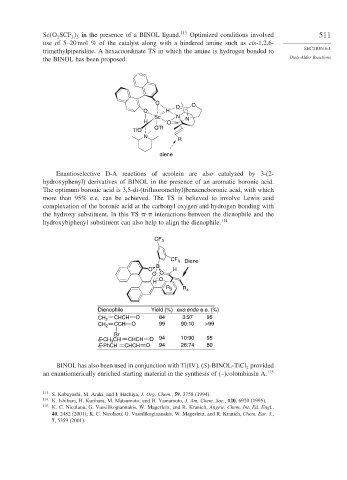
Sc O SCF in the presence of a BINOL ligand. 113 Optimized conditions involved 511
3 3 3
use of 5–20 mol % of the catalyst along with a hindered amine such as cis-1,2,6-
SECTION 6.1
trimethylpiperidine. A hexacoordinate TS in which the amine is hydrogen bonded to
the BINOL has been proposed. Diels-Alder Reactions
O O
O
O H
Sc N N
H O
OTf
TfO
N
R
diene
Enantioselective D-A reactions of acrolein are also catalyzed by 3-(2-
hydroxyphenyl) derivatives of BINOL in the presence of an aromatic boronic acid.
The optimum boronic acid is 3,5-di-(trifluoromethyl)benzeneboronic acid, with which
more than 95% e.e. can be achieved. The TS is believed to involve Lewis acid
complexation of the boronic acid at the carbonyl oxygen and hydrogen bonding with
the hydroxy substituent. In this TS - interactions between the dienophile and the
hydroxybiphenyl substituent can also help to align the dienophile. 114
CF 3
CF 3 Diene
B
O H
O O
O
H
R 3 R 4
Dienophile Yield (%) exo:endo e.e. (%)
CH 2 CHCH O 84 3:97 95
CH 2 CCH O 99 90:10 >99
Br
E-CH CH CHCH O 94 10:90 95
3
E-PhCH CHCH O 94 26:74 80
BINOL has also been used in conjunction with Ti(IV). (S)-BINOL-TiCl provided
2
an enantiomerically enriched starting material in the synthesis of (–)colombiasin A. 115
113
S. Kobayashi, M. Araki, and I. Hachiya, J. Org. Chem., 59, 3758 (1994).
114 K. Ishihara, H. Kurihara, M. Matsumoto, and H. Yamamoto, J. Am. Chem. Soc., 120, 6920 (1995).
115
K. C. Nicolaou, G. Vassilikogiannakis, W. Magerlein, and R. Kranich, Angew. Chem. Int. Ed. Engl.,
40, 2482 (2001); K. C. Nicolaou, G. Vassilikogiannakis, W. Magerlein, and R. Kranich, Chem. Eur. J.,
7, 5359 (2001).