Page 539 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 539
513
2.399
Cl 155.1
1.794 SECTION 6.1
76.7 O Diels-Alder Reactions
Ti 97.8
2.231
1.754 O
1.248 Cl
2.190
2.352
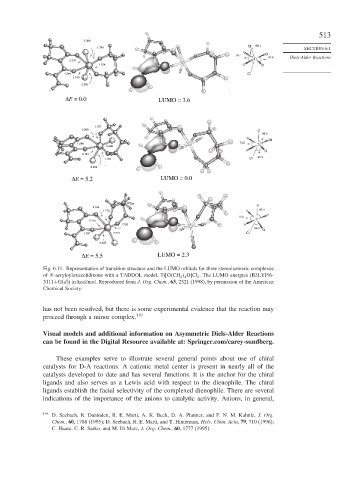
ΔE = 0.0 LUMO = 3.6
1.787
2.288
96.3
Ol
1.248 74.5 Ti
2.280
O
2.164
1.753 Cl 95.3
2.462
ΔE = 5.2 LUMO = 0.0
2.154
1.779 95.4
75.5
Ti
2.214
1.750
96.3
Cl
1.252 2.271 Cl
2.435
ΔE = 5.5 LUMO = 2.3
Fig. 6.11. Representation of transition structure and the LUMO orbitals for three stereoisomeric complexes
of N-acryloyloxazolidinone with a TADDOL model, Ti O CH 2 4 O Cl 2 . The LUMO energies (B3LYP/6-
3111+G(d)) in kcal/mol. Reproduced from J. Org. Chem., 63, 2321 (1998), by permission of the American
Chemical Society.
has not been resolved, but there is some experimental evidence that the reaction may
proceed through a minor complex. 119
Visual models and additional information on Asymmetric Diels-Alder Reactions
can be found in the Digital Resource available at: Springer.com/carey-sundberg.
These examples serve to illustrate several general points about use of chiral
catalysts for D-A reactions. A cationic metal center is present in nearly all of the
catalysts developed to date and has several functions. It is the anchor for the chiral
ligands and also serves as a Lewis acid with respect to the dienophile. The chiral
ligands establish the facial selectivity of the complexed dienophile. There are several
indications of the importance of the anions to catalytic activity. Anions, in general,
119
D. Seebach, R. Dahinden, R. E. Marti, A. K. Beck, D. A. Plattner, and F. N. M. Kuhnle, J. Org.
Chem., 60, 1788 (1995); D. Seebach, R. E. Marti, and T. Hinterman, Helv. Chim. Acta, 79, 710 (1996);
C. Haase, C. R. Sarko, and M. Di Mare, J. Org. Chem., 60, 1777 (1995).