Page 541 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 541
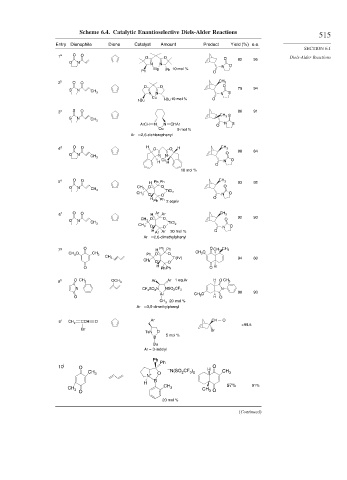
Scheme 6.4. Catalytic Enantioselective Diels-Alder Reactions
515
Entry Dienophile Diene Catalyst Amount Product Yield (%) e.e.
SECTION 6.1
1 a O O O O O 82 95 Diels-Alder Reactions
O N N N O
Mg Ph 10 mol % N
Ph O
b CH 3
2 O O
O O O 79 94
S N
CH 3
N N N S
Cu
t-Bu t-Bu 10 mol % O
c S O
3 86 91
CH 3 S
S N
CH 3
ArCH N N CHAr O N S
Cu 9 mol %
Ar 2,6-dichlorophenyl
d O O H CH 3
4 O O H
O 88 84
O N N N
CH 3
Cu N O
H H
O
10 mol %
5 e O O H Ph Ph CH 3 93 92
O N CH 3 O O O
CH 3
TiCl 2
CH 3 N O
O O
H Ph Ph 2 equiv O
f O O Ar Ar CH 3
6 H
O O O 92 93
O N
CH 3
CH 3 TiCl 2
O O N O
CH 3
H Ar Ar 20 mol % O
Ar 2,6-dimethylphenyl
g O Ph Ph
7 H O CH 3 CH 3
CH O CH 3 Ph O O CH 3 O
3
CH 3 Ti(IV) 94 80
CH 3 O O
O H Ph Ph O H
8 h O CH 3 OCH 3 Ar Ar 1 equiv H O CH 3
N CF 3 SO 2 N NSO 2 CF 3 N
98 93
Al CH 3 O
O H O
CH 3 20 mol %
Ar 3,5-dimethylphenyl
9 i CH CCH O Ar CH O
2
>99.5
Br Br
TsN O
5 mol %
B
Bu
Ar = 3-indolyl
Ph
Ph
10 j O O
– N(SO 2 CF 3 ) 2 H
CH 3 O CH 3
N +
B
H 97%
CH 3 91%
CH 3 CH 3 O
O
20 mol %
(Continued)