Page 534 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 534
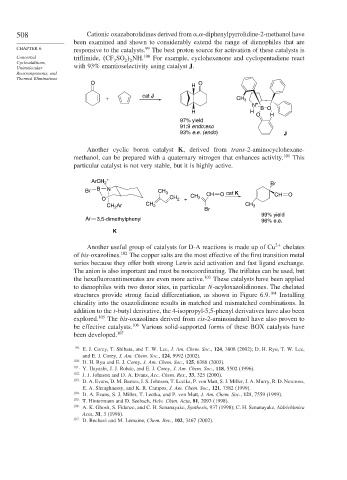
508 Cationic oxazaborolidines derived from , -diphenylpyrrolidine-2-methanol have
been examined and shown to considerably extend the range of dienophiles that are
99
CHAPTER 6 responsive to the catalysts. The best proton source for activation of these catalysts is
Concerted triflimide, CF SO NH. 100 For example, cyclohexenone and cyclopentadiene react
Cycloadditions, 3 2 2
Unimolecular with 93% enantioselectivity using catalyst J.
Rearrangements, and
Thermal Eliminations
O O
H
cat J
+ CH 3
N +
H H B O
O H
97% yield
91:9 endo:exo
93% e.e. (endo) J
Another cyclic boron catalyst K, derived from trans-2-aminocyclohexane-
methanol, can be prepared with a quaternary nitrogen that enhances activity. 101 This
particular catalyst is not very stable, but it is highly active.
+
ArCH 2
Br
Br B N CH 3 CH O cat K CH O
O CH 2 + CH 2
CH 2 Ar CH 2 CH 3
Br
99% yield
Ar 3,5-dimethylphenyl 96% e.e.
K
Another useful group of catalysts for D-A reactions is made up of Cu 2+ chelates
of bis-oxazolines. 102 The copper salts are the most effective of the first transition metal
series because they offer both strong Lewis acid activation and fast ligand exchange.
The anion is also important and must be noncoordinating. The triflates can be used, but
the hexafluoroantimonates are even more active. 103 These catalysts have been applied
to dienophiles with two donor sites, in particular N-acyloxazolidinones. The chelated
structures provide strong facial differentiation, as shown in Figure 6.9. 104 Installing
chirality into the oxazolidinone results in matched and mismatched combinations. In
addition to the t-butyl derivative, the 4-isopropyl-5,5-phenyl derivatives have also been
explored. 105 The bis-oxazolines derived from cis-2-aminoindanol have also proven to
be effective catalysts. 106 Various solid-supported forms of these BOX catalysts have
been developed. 107
99
E. J. Corey, T. Shibata, and T. W. Lee, J. Am. Chem. Soc., 124, 3808 (2002); D. H. Ryu, T. W. Lee,
and E. J. Corey, J. Am. Chem. Soc., 124, 9992 (2002).
100 D. H. Ryu and E. J. Corey, J. Am. Chem. Soc., 125, 6388 (2003).
101 Y. Hayashi, J. J. Rohde, and E. J. Corey, J. Am. Chem. Soc., 118, 5502 (1996).
102
J. J. Johnson and D. A. Evans, Acc. Chem. Res., 33, 325 (2000).
103 D. A. Evans, D. M. Barnes, J. S. Johnson, T. Lectka, P. von Matt, S. J. Miller, J. A. Murry, R. D. Norcross,
E. A. Shaughnessy, and K. R. Campos, J. Am. Chem. Soc., 121, 7582 (1999).
104
D. A. Evans, S. J. Miller, T. Lectka, and P. von Matt, J. Am. Chem. Soc., 121, 7559 (1999).
105 T. Hintermann and D. Seebach, Helv. Chim. Acta, 81, 2093 (1998).
106 A. K. Ghosh, S. Fidanze, and C. H. Senanayake, Synthesis, 937 (1998); C. H. Senanayake, Aldrichimica
Acta, 31, 3 (1998).
107
D. Rechavi and M. Lemaine, Chem. Rev., 102, 3467 (2002).