Page 494 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 494
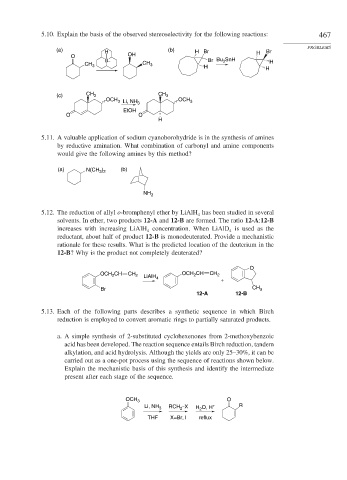
5.10. Explain the basis of the observed stereoselectivity for the following reactions: 467
PROBLEMS
(a) (b)
H H Br H Br
O OH
B– Br Bu SnH H
3
CH 3 CH 3
H H
(c) CH 3 CH 3
OCH OCH
3 Li, NH 3 3
EtOH
O O
H
5.11. A valuable application of sodium cyanoborohydride is in the synthesis of amines
by reductive amination. What combination of carbonyl and amine components
would give the following amines by this method?
(a) N(CH ) (b)
3 2
NH 2
5.12. The reduction of allyl o-bromphenyl ether by LiAlH has been studied in several
4
solvents. In ether, two products 12-A and 12-B are formed. The ratio 12-A:12-B
increases with increasing LiAlH concentration. When LiAlD is used as the
4
4
reductant, about half of product 12-B is monodeuterated. Provide a mechanistic
rationale for these results. What is the predicted location of the deuterium in the
12-B? Why is the product not completely deuterated?
O
OCH CH CH 2 LiAlH OCH CH CH 2
2
2
4
+
Br CH 3
12-A 12-B
5.13. Each of the following parts describes a synthetic sequence in which Birch
reduction is employed to convert aromatic rings to partially saturated products.
a. A simple synthesis of 2-substituted cyclohexenones from 2-methoxybenzoic
acid has been developed. The reaction sequence entails Birch reduction, tandem
alkylation, and acid hydrolysis. Although the yields are only 25–30%, it can be
carried out as a one-pot process using the sequence of reactions shown below.
Explain the mechanistic basis of this synthesis and identify the intermediate
present after each stage of the sequence.
OCH 3 O
Li, NH 3 RCH –X H O, H + R
2
2
THF X=Br, I reflux