Page 695 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 695
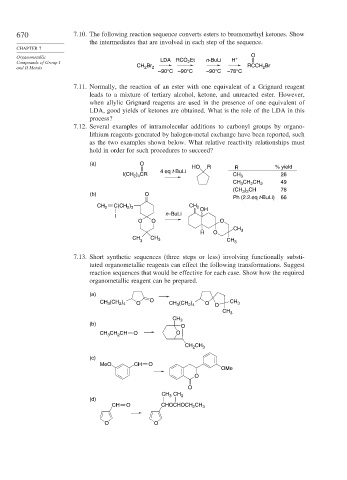
670 7.10. The following reaction sequence converts esters to bromomethyl ketones. Show
the intermediates that are involved in each step of the sequence.
CHAPTER 7
Organometallic + O
2
Compounds of Group I LDA RCO Et n-BuLi H
2
and II Metals CH Br 2 RCCH Br
2
–90°C –90°C –90°C –78°C
7.11. Normally, the reaction of an ester with one equivalent of a Grignard reagent
leads to a mixture of tertiary alcohol, ketone, and unreacted ester. However,
when allylic Grignard reagents are used in the presence of one equivalent of
LDA, good yields of ketones are obtained. What is the role of the LDA in this
process?
7.12. Several examples of intramolecular additions to carbonyl groups by organo-
lithium reagents generated by halogen-metal exchange have been reported, such
as the two examples shown below. What relative reactivity relationships must
hold in order for such procedures to succeed?
(a) O
HO R R % yield
4 eq t-BuLi
I(CH ) CR CH 3 26
2 4
CH CH CH 2 49
3
2
(CH ) CH 78
3 2
(b) O
Ph (2.2.eq t-BuLi) 66
CH 2 C(CH ) CH 2
2 3
OH
n -BuLi
I
O O O
CH
H O 3
CH CH 3
3 CH 3
7.13. Short synthetic sequences (three steps or less) involving functionally substi-
tuted organometallic reagents can effect the following transformations. Suggest
reaction sequences that would be effective for each case. Show how the required
organometallic reagent can be prepared.
(a)
CH (CH ) O O CH (CH ) O O CH 3
2 4
3
2 4
3
CH 3
CH 3
(b)
O
CH CH CH O O
2
3
CH 2 CH 3
(c)
MeO CH O
OMe
O
O
CH 3 CH 3
(d)
CH O CHOCHOCH CH 3
2
O O