Page 693 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 693
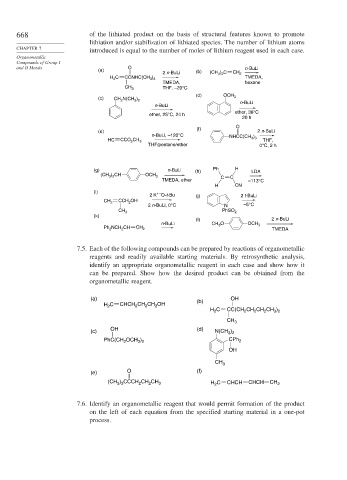
668 of the lithiated product on the basis of structural features known to promote
lithiation and/or stabilization of lithiated species. The number of lithium atoms
CHAPTER 7
introduced is equal to the number of moles of lithium reagent used in each case.
Organometallic
Compounds of Group I
and II Metals O n-BuLi
(a)
2 n-BuLi (b) (CH 3 ) 2 C CH 2
H 2 C CCNHC(CH 3 ) 3 TMEDA,
TMEDA, hexane
THF, –20°C
CH 3
(d)
(c) CH 2 N(CH 3 ) 2 OCH 3
n-BuLi
n-BuLi
ether, 38°C
ether, 25°C, 24 h
20 h
O
(f)
(e) 2 n-BuLi
n-BuLi, –120°C
HC CCO 2 CH 3 NHCC(CH 3 ) 3 THF,
THF/pentane/ether 0°C, 2 h
(g) n-BuLi (h) Ph H LDA
(CH 3 ) 2 CH OCH 3 C
TMEDA, ether C –113°C
H CN
(i)
+ –
2 K O-t-Bu (j) 2 t-BuLi
CCH 2 OH
CH 2
2 n-BuLi, 0°C N –5°C
CH 3 PhSO 2
(k)
(l) 2 n-BuLi
n-BuLi CH 3 O OCH 3
Ph 2 NCH 2 CH
TMEDA
CH 2
7.5. Each of the following compounds can be prepared by reactions of organometallic
reagents and readily available starting materials. By retrosynthetic analysis,
identify an appropriate organometallic reagent in each case and show how it
can be prepared. Show how the desired product can be obtained from the
organometallic reagent.
(a) (b) OH
H C CHCH 2 CH CH OH
2
2
2
H C CC(CH CH CH CH )
2
2
2
3 2
2
CH 3
OH (d)
(c) N(CH )
3 2
PhC(CH OCH ) CPh 2
3 2
2
OH
CH 3
(e) O (f)
(CH ) CCCH CH CH 3 H C CHCH CHCH CH 2
2
3 3
2
2
7.6. Identify an organometallic reagent that would permit formation of the product
on the left of each equation from the specified starting material in a one-pot
process.