Page 694 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 694
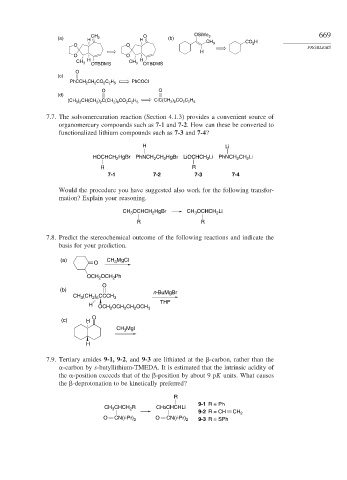
(a) H CH 2 H O (b) OSiMe 3 669
CH 3 CO 2 H
O O
PROBLEMS
H
O O
H H
CH 3 CH 3
OTBDMS OTBDMS
O
(c)
PhCOCl
PhCCH 2 CH 2 CO 2 C 2 H 5
O O
(d)
(CH 3 ) 2 CH(CH 2 ) 2 C(CH 2 ) 6 CO 2 C 2 H 5 ClC(CH 2 ) 6 CO 2 C 2 H 5
7.7. The solvomercuration reaction (Section 4.1.3) provides a convenient source of
organomercury compounds such as 7-1 and 7-2. How can these be converted to
functionalized lithium compounds such as 7-3 and 7-4?
H Li
HOCHCH HgBr PhNCH CH HgBr LiOCHCH Li PhNCH CH Li
2
2
2
2
2
2
R R
7-1 7-2 7-3 7-4
Would the procedure you have suggested also work for the following transfor-
mation? Explain your reasoning.
CH OCHCH HgBr CH OCHCH Li
3
2
2
3
R R
7.8. Predict the stereochemical outcome of the following reactions and indicate the
basis for your prediction.
(a) CH 3 MgCl
O
OCH OCH Ph
2
2
O
(b)
n-BuMgBr
CH (CH ) CCCH 3
3
2 6
THF
H OCH OCH CH OCH 3
2
2
2
O
(c) H
MgI
CH 3
H
7.9. Tertiary amides 9-1, 9-2, and 9-3 are lithiated at the
-carbon, rather than the
-carbon by s-butyllithium-TMEDA. It is estimated that the intrinsic acidity of
the
-position exceeds that of the
-position by about 9 pK units. What causes
the
-deprotonation to be kinetically preferred?
R
9-1 R = Ph
CH CHCH R CH3CHCHLi
3
2
9-2 R = CH CH 2
O CN(i-Pr) 2 O CN(i-Pr) 2 9-3 R = SPh