Page 689 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 689
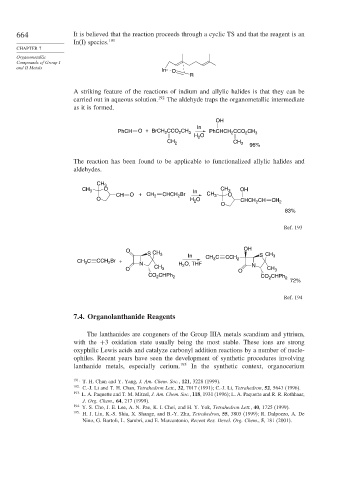
664 It is believed that the reaction proceeds through a cyclic TS and that the reagent is an
In(I) species. 191
CHAPTER 7
Organometallic
Compounds of Group I
and II Metals
In O
R
A striking feature of the reactions of indium and allylic halides is that they can be
carried out in aqueous solution. 192 The aldehyde traps the organometallic intermediate
as it is formed.
OH
In
PhCH O + BrCH CCO CH 3 PhCHCH CCO CH 3
2
2
2
2
H 2 O
CH 2 CH 2 96%
The reaction has been found to be applicable to functionalized allylic halides and
aldehydes.
CH 3
CH 3 O In CH 3 OH
CH O + CH 2 CHCH Br CH 3 O
2
O H O CHCH CH CH
2
O 2 2
83%
Ref. 193
O OH
S CH 3
In C CCH S CH 3
CH C CCH Br + N H O, THF CH 3 2
2
3
O CH 3 2 O N CH 3
CO CHPh 2 CO CHPh 2
2
2
72%
Ref. 194
7.4. Organolanthanide Reagents
The lanthanides are congeners of the Group IIIA metals scandium and yttrium,
with the +3 oxidation state usually being the most stable. These ions are strong
oxyphilic Lewis acids and catalyze carbonyl addition reactions by a number of nucle-
ophiles. Recent years have seen the development of synthetic procedures involving
lanthanide metals, especially cerium. 195 In the synthetic context, organocerium
191 T. H. Chan and Y. Yang, J. Am. Chem. Soc., 121, 3228 (1999).
192
C.-J. Li and T. H. Chan, Tetrahedron Lett., 32, 7017 (1991); C.-J. Li, Tetrahedron, 52, 5643 (1996).
193
L. A. Paquette and T. M. Mitzel, J. Am. Chem. Soc., 118, 1931 (1996); L. A. Paquette and R. R. Rothhaar,
J. Org. Chem., 64, 217 (1999).
194 Y. S. Cho, J. E. Lee, A. N. Pae, K. I. Choi, and H. Y. Yok, Tetrahedron Lett., 40, 1725 (1999).
195
H. J. Liu, K.-S. Shia, X. Shange, and B.-Y. Zhu, Tetrahedron, 55, 3803 (1999); R. Dalpozzo, A. De
Nino, G. Bartoli, L. Sambri, and E. Marcantonio, Recent Res. Devel. Org. Chem., 5, 181 (2001).