Page 685 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 685
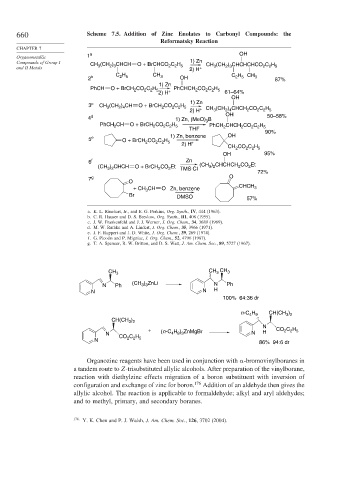
660 Scheme 7.5. Addition of Zinc Enolates to Carbonyl Compounds: the
Reformatsky Reaction
CHAPTER 7
Organometallic 1 a OH
Compounds of Group I CH (CH ) CHCH O + BrCHCO C H 1) Zn CH (CH ) CHCHCHCO C H
and II Metals 3 2 3 2 2 5 2) H + 3 2 3 2 2 5
C H CH C H
2 b 2 5 3 OH 2 5 CH 3 87%
1) Zn
PhCH O + BrCH CO C H PhCHCH CO C H
2 2 5
2 2 5
2
2
2) H + 61–64%
OH
3 c CH 3 (CH ) CH O + BrCH CO C H 1) Zn
2
2 2 5
2 4
2 4
2 2 5
2
2) H + CH (CH ) CHCH CO C H
3
OH
4 d 1) Zn, (MeO) B 50–58%
3
PhCH CH O + BrCH CO C H PhCH CHCH CO C H
2
2 2 5
2
THF 2 2 2 2 5
90%
5 e O + BrCH CO C H 1) Zn, benzene OH
2 2 5
2
2) H + CH CO C H
2
2 2 5
OH 95%
6 f Zn
2
(CH ) CHCH O + BrCH 2 CO Et TMS CI (CH ) CHCHCH CO Et
2
3 2
2
3 2
72%
7 g O
O
+ CH CH O Zn, benzene CHCH 3
3
Br DMSO 57%
a. K. L. Rinehart, Jr., and E. G. Perkins, Org. Synth., IV, 444 (1963).
b. C. R. Hauser and D. S. Breslow, Org. Synth., III, 408 (1955).
c. J. W. Frankenfeld and J. J. Werner, J. Org. Chem., 34, 3689 (1969).
d. M. W. Rathke and A. Lindert, J. Org. Chem., 35, 3966 (1971).
e. J. F. Ruppert and J. D. White, J. Org. Chem., 39, 269 (1974).
f. G. Picotin and P. Migniac, J. Org. Chem., 52, 4796 (1987).
g. T. A. Spencer, R. W. Britton, and D. S. Watt, J. Am. Chem. Soc., 89, 5727 (1967).
CH 3 CH 3 CH 3
) ZnLi
N Ph (CH 3 3 N Ph
N N H
100% 64:36 dr
CH(CH )
n- C 4 H 9 3 2
)
CH(CH 3 2
N
+ (n -C 4 H 9 ) 3 ZnMgBr CO C H
2 2 5
N CO C H N H
N 2 2 5 86% 94:6 dr
Organozinc reagents have been used in conjunction with
-bromovinylboranes in
a tandem route to Z-trisubstituted allylic alcohols. After preparation of the vinylborane,
reaction with diethylzinc effects migration of a boron substituent with inversion of
configuration and exchange of zinc for boron. 176 Addition of an aldehyde then gives the
allylic alcohol. The reaction is applicable to formaldehyde; alkyl and aryl aldehydes;
and to methyl, primary, and secondary boranes.
176
Y. K. Chen and P. J. Walsh, J. Am. Chem. Soc., 126, 3702 (2004).