Page 683 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 683
658
CHAPTER 7
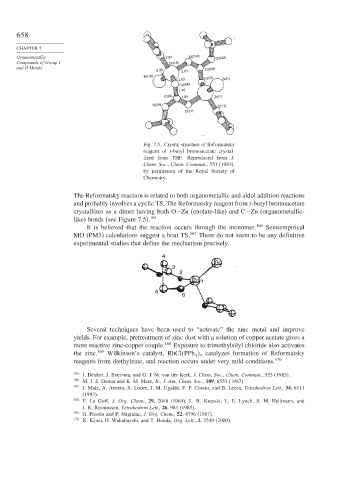
Organometallic 2.07 O(21A) O(24A)
Compounds of Group I Zn(1A)
and II Metals C(22A)
2.35 2.01
Br(1A) C(23)
2.03 Br(1)
C(23A)
1.45
C(22) 1.24 Zn(1)
O(24) O(11)
O(21)
Fig. 7.5. Crystal structure of Reformatsky
reagent of t-butyl bromoacetate crystal-
lized from THF. Reproduced from J.
Chem. Soc., Chem. Commun., 553 (1983),
by permission of the Royal Society of
Chemistry.
The Reformatsky reaction is related to both organometallic and aldol addition reactions
and probably involves a cyclic TS. The Reformatsky reagent from t-butyl bromoacetate
crystallizes as a dimer having both O−Zn (enolate-like) and C−Zn (organometallic-
like) bonds (see Figure 7.5). 165
It is believed that the reaction occurs through the monomer. 166 Semiempirical
MO (PM3) calculations suggest a boat TS. 167 There do not seem to be any definitive
experimental studies that define the mechanism precisely.
4
Br
3
2
Zn 1
5
6
Several techniques have been used to “activate” the zinc metal and improve
yields. For example, pretreatment of zinc dust with a solution of copper acetate gives a
more reactive zinc-copper couple. 168 Exposure to trimethylsilyl chloride also activates
the zinc. 169 Wilkinson’s catalyst, RhCl PPh catalyzes formation of Reformatsky
3 3
reagents from diethylzinc, and reaction occurs under very mild conditions. 170
165 J. Dekker, J. Boersma, and G. J. M. van der Kerk, J. Chem. Soc., Chem. Commun., 553 (1983).
166 M. J. S. Dewar and K. M. Merz, Jr., J. Am. Chem. Soc., 109, 6553 (1987).
167
J. Maiz, A. Arrieta, X. Lopez, J. M. Ugalde, F. P. Cossio, and B. Lecea, Tetrahedron Lett., 34, 6111
(1993).
168 E. Le Goff, J. Org. Chem., 29, 2048 (1964); L. R. Krepski, L. E. Lynch, S. M. Heilmann, and
J. K. Rasmussen, Tetrahedron Lett., 26, 981 (1985).
169 G. Picotin and P. Miginiac, J. Org. Chem., 52, 4796 (1987).
170
K. Kanai, H. Wakabayshi, and T. Honda, Org. Lett., 2, 2549 (2000).