Page 687 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 687
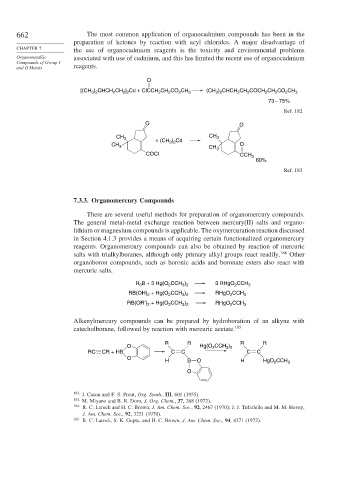
662 The most common application of organocadmium compounds has been in the
preparation of ketones by reaction with acyl chlorides. A major disadvantage of
CHAPTER 7 the use of organocadmium reagents is the toxicity and environmental problems
Organometallic associated with use of cadmium, and this has limited the recent use of organocadmium
Compounds of Group I
and II Metals reagents.
O
[(CH ) CHCH CH ] Cd + ClCCH CH CO CH 3 (CH ) CHCH CH COCH CH CO CH 3
2
3 2
2
2
3 2
2
2
2
2
2 2
2
2
73 – 75%
Ref. 182
O O
CH 3 CH 3
) Cd
+ (CH 3 2
CH 3 CH 3 O
COCl CCH 3
60%
Ref. 183
7.3.3. Organomercury Compounds
There are several useful methods for preparation of organomercury compounds.
The general metal-metal exchange reaction between mercury(II) salts and organo-
lithium or magnesium compounds is applicable. The oxymercuration reaction discussed
in Section 4.1.3 provides a means of acquiring certain functionalized organomercury
reagents. Organomercury compounds can also be obtained by reaction of mercuric
salts with trialkylboranes, although only primary alkyl groups react readily. 184 Other
organoboron compounds, such as boronic acids and boronate esters also react with
mercuric salts.
R B + 3 Hg(O CCH ) 3 RHgO CCH 3
3
2
3 2
2
RB(OH) + Hg(O CCH ) RHgO CCH 3
2
3 2
2
2
RB(OR′) + Hg(O CCH ) RHgO CCH 3
3 2
2
2
2
Alkenylmercury compounds can be prepared by hydroboration of an alkyne with
catecholborane, followed by reaction with mercuric acetate. 185
R R R R
O Hg(O CCH )
2
3 2
RC CR + HB C C C C
O
H B O H HgO 2 CCH 3
O
182
J. Cason and F. S. Prout, Org. Synth., III, 601 (1955).
183 M. Miyano and B. R. Dorn, J. Org. Chem., 37, 268 (1972).
184 R. C. Larock and H. C. Brown, J. Am. Chem. Soc., 92, 2467 (1970); J. J. Tufariello and M. M. Hovey,
J. Am. Chem. Soc., 92, 3221 (1970).
185
R. C. Larock, S. K. Gupta, and H. C. Brown, J. Am. Chem. Soc., 94, 4371 (1972).