Page 688 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 688
The organomercury compounds can be used in situ or isolated as organomercuric 663
halides.
Organomercury compounds are weak nucleophiles and react only with very SECTION 7.3
reactive electrophiles. They readily undergo electrophilic substitution by halogens. Organometallic
Compounds of Group
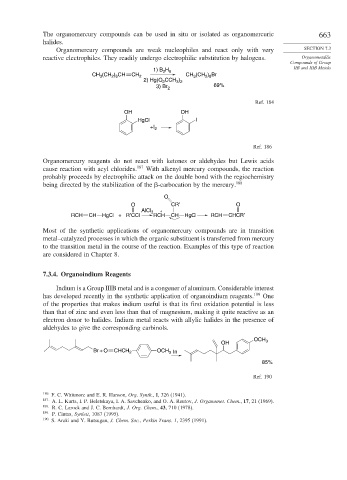
1) B 2 H 6 IIB and IIIB Metals
CH (CH ) CH CH 2 CH (CH ) Br
2 8
3
2 6
3
2) Hg(O CCH )
3 2
2
3) Br 2 69%
Ref. 184
OH OH
HgCl I
+I
2
Ref. 186
Organomercury reagents do not react with ketones or aldehydes but Lewis acids
cause reaction with acyl chlorides. 187 With alkenyl mercury compounds, the reaction
probably proceeds by electrophilic attack on the double bond with the regiochemistry
being directed by the stabilization of the
-carbocation by the mercury. 188
O
O CR′ O
AlCl 3 +
RCH CH HgCl + R′CCl RCH CH HgCl RCH CHCR′
Most of the synthetic applications of organomercury compounds are in transition
metal–catalyzed processes in which the organic substituent is transferred from mercury
to the transition metal in the course of the reaction. Examples of this type of reaction
are considered in Chapter 8.
7.3.4. Organoindium Reagents
Indium is a Group IIIB metal and is a congener of aluminum. Considerable interest
has developed recently in the synthetic application of organoindium reagents. 189 One
of the properties that makes indium useful is that its first oxidation potential is less
than that of zinc and even less than that of magnesium, making it quite reactive as an
electron donor to halides. Indium metal reacts with allylic halides in the presence of
aldehydes to give the corresponding carbinols.
OCH 3
OH
Br + O CHCH 2 OCH In
3
85%
Ref. 190
186 F. C. Whitmore and E. R. Hanson, Org. Synth., I, 326 (1941).
187
A. L. Kurts, I. P. Beletskaya, I. A. Savchenko, and O. A. Reutov, J. Organomet. Chem., 17, 21 (1969).
188
R. C. Larock and J. C. Bernhardt, J. Org. Chem., 43, 710 (1978).
189 P. Cintas, Synlett, 1087 (1995).
190
S. Araki and Y. Butsugan, J. Chem. Soc., Perkin Trans. 1, 2395 (1991).