Page 686 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 686
– H R′ 661
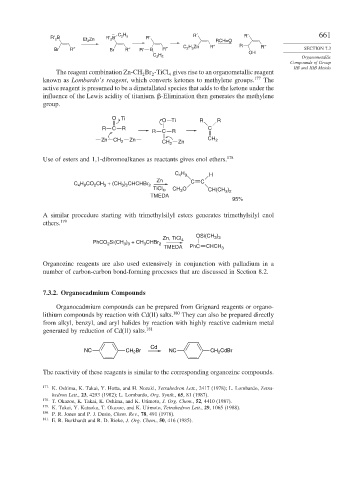
R′ B R′ 2 B C 2 5 R′ R′
2 Zn
Et 2 RCH=O
5
Br R″ Br R″ R′ B R″ C 2 H Zn R″ R R″ SECTION 7.3
H OH
C 2 5 Organometallic
Compounds of Group
IIB and IIIB Metals
The reagent combination Zn-CH Br -TiCl gives rise to an organometallic reagent
2 2 4
known as Lombardo’s reagent, which converts ketones to methylene groups. 177 The
active reagent is presumed to be a dimetallated species that adds to the ketone under the
influence of the Lewis acidity of titanium.
-Elimination then generates the methylene
group.
O Ti O Ti R R
R C R C
R C R
Zn CH 2 Zn CH 2
CH 2 Zn
Use of esters and 1,1-dibromoalkanes as reactants gives enol ethers. 178
C H 9 H
4
Zn C C
C H CO CH + (CH ) CHCHBr 2
3 2
3
2
4 9
TiCl , CH O CH(CH 3 2
)
3
4
TMEDA
95%
A similar procedure starting with trimethylsilyl esters generates trimethylsilyl enol
ethers. 179
)
Zn, TiCl 4 OSi(CH 3 3
PhCO Si(CH ) + CH CHBr 2
3
2
3 3
TMEDA PhC CHCH 3
Organozinc reagents are also used extensively in conjunction with palladium in a
number of carbon-carbon bond-forming processes that are discussed in Section 8.2.
7.3.2. Organocadmium Compounds
Organocadmium compounds can be prepared from Grignard reagents or organo-
lithium compounds by reaction with Cd(II) salts. 180 They can also be prepared directly
from alkyl, benzyl, and aryl halides by reaction with highly reactive cadmium metal
generated by reduction of Cd(II) salts. 181
Cd
NC CH 2 Br NC CH CdBr
2
The reactivity of these reagents is similar to the corresponding organozinc compounds.
177 K. Oshima, K. Takai, Y. Hotta, and H. Nozaki, Tetrahedron Lett., 2417 (1978); L. Lombardo, Tetra-
hedron Lett., 23, 4293 (1982); L. Lombardo, Org. Synth., 65, 81 (1987).
178
T. Okazoe, K. Takai, K. Oshima, and K. Utimoto, J. Org. Chem., 52, 4410 (1987).
179
K. Takai, Y. Kataoka, T. Okazoe, and K. Utimoto, Tetrahedron Lett., 29, 1065 (1988).
180 P. R. Jones and P. J. Desio, Chem. Rev., 78, 491 (1978).
181
E. R. Burkhardt and R. D. Rieke, J. Org. Chem., 50, 416 (1985).