Page 682 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 682
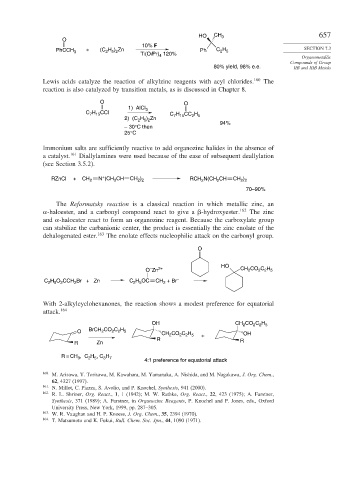
HO CH 3 657
O
10% F
H
PhCCH 3 + (C H ) Zn Ph C 2 5 SECTION 7.3
2 5 2
Ti(Oi Pr) 4 120%
Organometallic
Compounds of Group
80% yield, 98% e.e. IIB and IIIB Metals
Lewis acids catalyze the reaction of alkylzinc reagents with acyl chlorides. 160 The
reaction is also catalyzed by transition metals, as is discussed in Chapter 8.
O O
1) AlCl 3
C H CCl H CC H
7 15
2) (C H ) Zn C 7 15 2 5
2 5 2
94%
– 30°C then
25°C
Immonium salts are sufficiently reactive to add organozinc halides in the absence of
a catalyst. 161 Diallylamines were used because of the ease of subsequent deallylation
(see Section 3.5.2).
+
RZnCl + CH 2 N (CH CH CH ) RCH N(CH CH CH )
2 2
2
2
2 2
2
70–90%
The Reformatsky reaction is a classical reaction in which metallic zinc, an
-haloester, and a carbonyl compound react to give a
-hydroxyester. 162 The zinc
and
-haloester react to form an organozinc reagent. Because the carboxylate group
can stabilize the carbanionic center, the product is essentially the zinc enolate of the
dehalogenated ester. 163 The enolate effects nucleophilic attack on the carbonyl group.
O
HO
–
O Zn 2+ CH CO C H
2 2 5
2
C H O CCH Br + Zn C H OC CH + Br –
2 5
2
2
2
2 5
With 2-alkylcyclohexanones, the reaction shows a modest preference for equatorial
attack. 164
OH CH 2 CO C H
2 2 5
O BrCH 2 CO C H CH CO C H + OH
2 2 5
2
2 2 5
R R
R Zn
R = CH , C H , C H
3 7
3
2 5
4:1 preference for equatorial attack
160 M. Arisawa, Y. Torisawa, M. Kawahara, M. Yamanaka, A. Nishida, and M. Nagakawa, J. Org. Chem.,
62, 4327 (1997).
161
N. Millot, C. Piazza, S. Avolio, and P. Knochel, Synthesis, 941 (2000).
162 R. L. Shriner, Org. React., 1, 1 (1942); M. W. Rathke, Org. React., 22, 423 (1975); A. Furstner,
Synthesis, 371 (1989); A. Furstner, in Organozinc Reagents, P. Knochel and P. Jones, eds., Oxford
University Press, New York, 1999, pp. 287–305.
163 W. R. Vaughan and H. P. Knoess, J. Org. Chem., 35, 2394 (1970).
164
T. Matsumoto and K. Fukui, Bull. Chem. Soc. Jpn., 44, 1090 (1971).