Page 697 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 697
672 However, with 15-A and 15-B, the regioselectivity is reversed.
CHAPTER 7 OH
CH CH 3 O CH 3
Organometallic 3 O O MgBr
CH 3 CH 3 CH 3 (CH ) COCH CH CH 3
Compounds of Group I 3 3 2
and II Metals O O O
15-A
NH 2 CH NH 2
O 3 MgBr
(CH ) COCH CH CH 3 CH 3 (CH ) COCH CHCHCH OC(CH )
2
2
3 3
3 3
3 3
2
O
15-B OH
What factors might lead to the reversal in regioselectivity?
7.16. List several features of organocerium reagents that make them applicable to
specific synthetic transformations. Give a specific example illustrating each
feature.
7.17. Normally, organometallic reagents with potential leaving groups in the
-position decompose readily by elimination. Two examples of reagents with
greater stability are described below. Indicate what structural feature(s) may be
contributing to the relative stability of these reagents.
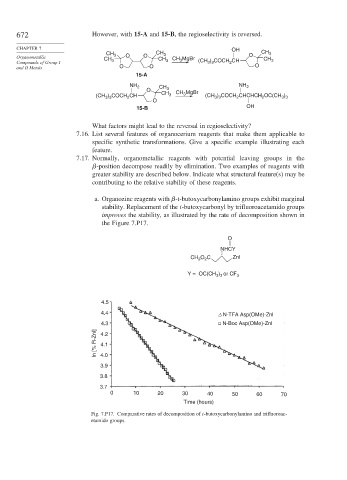
a. Organozinc reagents with -t-butoxycarbonylamino groups exhibit marginal
stability. Replacement of the t-butoxycarbonyl by trifluoroacetamido groups
improves the stability, as illustrated by the rate of decomposition shown in
the Figure 7.P17.
O
NHCY
CH O C ZnI
2
3
Y = OC(CH ) or CF 3
3 3
4.5
4.4 Δ N-TFA Asp(OMe)-Znl
4.3 N-Boc Asp(OMe)-Znl
In [% R-Znl] 4.2
4.1
4.0
3.9
3.8
3.7
0 10 20 30 40 50 60 70
Time (hours)
Fig. 7.P17. Comparative rates of decomposition of t-butoxycarbonylamino and trifluoroac-
etamido groups.