Page 701 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 701
677
C6 C7
SECTION 8.1
C5
Organocopper
C8
Cu1 Cu1 Intermediates
C4 C9
Cu2” Cu2’
Cu2 Cu3 Cu4
Cu2
C15
C10
Li3 Mg
C14
C11
C12
C13
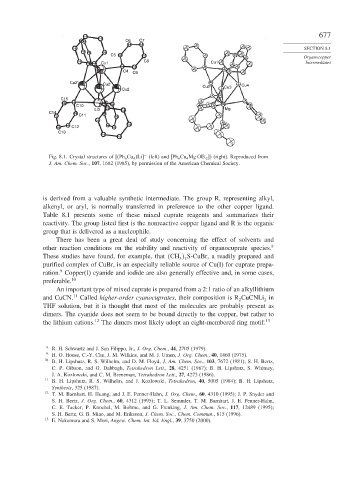
Fig. 8.1. Crystal structures of Ph 6 Cu 4 Li
(left) and Ph 6 Cu 4 Mg OEt 2
(right). Reproduced from
−
J. Am. Chem. Soc., 107, 1682 (1985), by permission of the American Chemical Society.
is derived from a valuable synthetic intermediate. The group R, representing alkyl,
alkenyl, or aryl, is normally transferred in preference to the other copper ligand.
Table 8.1 presents some of these mixed cuprate reagents and summarizes their
reactivity. The group listed first is the nonreactive copper ligand and R is the organic
group that is delivered as a nucleophile.
There has been a great deal of study concerning the effect of solvents and
other reaction conditions on the stability and reactivity of organocuprate species. 8
These studies have found, for example, that CH S-CuBr, a readily prepared and
3 2
purified complex of CuBr, is an especially reliable source of Cu(I) for cuprate prepa-
9
ration. Copper(I) cyanide and iodide are also generally effective and, in some cases,
preferable. 10
An important type of mixed cuprate is prepared from a 2:1 ratio of an alkyllithium
and CuCN. 11 Called higher-order cyanocuprates, their composition is R CuCNLi in
2 2
THF solution, but it is thought that most of the molecules are probably present as
dimers. The cyanide does not seem to be bound directly to the copper, but rather to
the lithium cations. 12 The dimers most likely adopt an eight-membered ring motif. 13
8 R. H. Schwartz and J. San Filippo, Jr., J. Org. Chem., 44, 2705 (1979).
9
H. O. House, C.-Y. Chu, J. M. Wilkins, and M. J. Umen, J. Org. Chem., 40, 1460 (1975).
10 B. H. Lipshutz, R. S. Wilhelm, and D. M. Floyd, J. Am. Chem. Soc., 103, 7672 (1981); S. H. Bertz,
C. P. Gibson, and G. Dabbagh, Tetrahedron Lett., 28, 4251 (1987); B. H. Lipshutz, S. Whitney,
J. A. Kozlowski, and C. M. Breneman, Tetrahedron Lett., 27, 4273 (1986).
11
B. H. Lipshutz, R. S. Wilhelm, and J. Kozlowski, Tetrahedron, 40, 5005 (1984); B. H. Lipshutz,
Synthesis, 325 (1987).
12 T. M. Barnhart, H. Huang, and J. E. Penner-Hahn, J. Org. Chem., 60, 4310 (1995); J. P. Snyder and
S. H. Bertz, J. Org. Chem., 60, 4312 (1995); T. L. Semmler, T. M. Barnhart, J. E. Penner-Hahn,
C. E. Tucker, P. Knochel, M. Bohme, and G. Frenking, J. Am. Chem. Soc., 117, 12489 (1995);
S. H. Bertz, G. B. Miao, and M. Eriksson, J. Chem. Soc., Chem. Commun., 815 (1996).
13
E. Nakamura and S. Mori, Angew. Chem. Int. Ed. Engl., 39, 3750 (2000).