Page 703 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 703
679
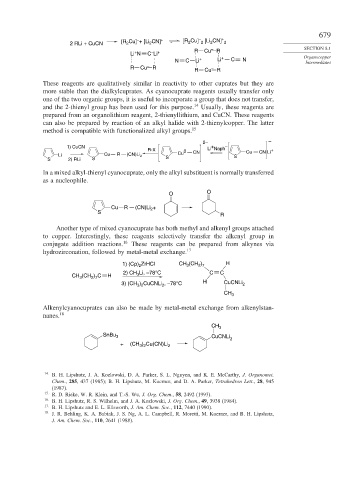
+
–
–
2
2
2
2 RLi + CuCN [R Cu] + [Li CN] + [R Cu] 2 [Li CN] 2 SECTION 8.1
2
–
+
Li N C Li + R Cu – R Organocopper
N C Li + Li + C N Intermediates
R Cu – R –
R Cu R
These reagents are qualitatively similar in reactivity to other cuprates but they are
more stable than the dialkylcuprates. As cyanocuprate reagents usually transfer only
one of the two organic groups, it is useful to incorporate a group that does not transfer,
and the 2-thienyl group has been used for this purpose. 14 Usually, these reagents are
prepared from an organolithium reagent, 2-thienyllithium, and CuCN. These reagents
can also be prepared by reaction of an alkyl halide with 2-thienylcopper. The latter
method is compatible with functionalized alkyl groups. 15
2– –
1) CuCN Li Naph –
+
R-X 0 Cu CN Li +
Li Cu R (CN)Li 2 Cu CN S
S 2) RLi S S
In a mixed alkyl-thienyl cyanocuprate, only the alkyl substituent is normally transferred
as a nucleophile.
O
O
Cu R (CN)Li 2 +
S
R
Another type of mixed cyanocuprate has both methyl and alkenyl groups attached
to copper. Interestingly, these reagents selectively transfer the alkenyl group in
conjugate addition reactions. 16 These reagents can be prepared from alkynes via
hydrozirconation, followed by metal-metal exchange. 17
1) (Cp) ZrHCl CH (CH ) H
3
2
2 7
2) CH Li, –78°C C C
CH (CH ) C H 3
3
2 7
3) (CH ) CuCNLi , –78°C H CuCNLi 2
3 2
2
CH 3
Alkenylcyanocuprates can also be made by metal-metal exchange from alkenylstan-
nanes. 18
CH 3
SnBu 3 CuCNLi
2
+ (CH ) Cu(CN)Li 2
3 2
14 B. H. Lipshutz, J. A. Kozlowski, D. A. Parker, S. L. Nguyen, and K. E. McCarthy, J. Organomet.
Chem., 285, 437 (1985); B. H. Lipshutz, M. Koerner, and D. A. Parker, Tetrahedron Lett., 28, 945
(1987).
15
R. D. Rieke, W. R. Klein, and T.-S. Wu, J. Org. Chem., 58, 2492 (1993).
16
B. H. Lipshutz, R. S. Wilhelm, and J. A. Kozlowski, J. Org. Chem., 49, 3938 (1984).
17 B. H. Lipshutz and E. L. Ellsworth, J. Am. Chem. Soc., 112, 7440 (1990).
18
J. R. Behling, K. A. Babiak, J. S. Ng, A. L. Campbell, R. Moretti, M. Koerner, and B. H. Lipshutz,
J. Am. Chem. Soc., 110, 2641 (1988).