Page 707 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 707
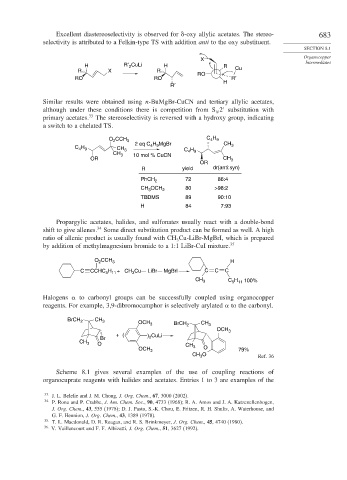
Excellent diastereoselectivity is observed for
-oxy allylic acetates. The stereo- 683
selectivity is attributed to a Felkin-type TS with addition anti to the oxy substituent.
SECTION 8.1
Organocopper
X Intermediates
H R′ 2 CuLi H R
R X R Cu
RO
RO RO R′
R′ H
Similar results were obtained using n-BuMgBr-CuCN and tertiary allylic acetates,
although under these conditions there is competition from S 2 substitution with
N
primary acetates. 33 The stereoselectivity is reversed with a hydroxy group, indicating
a switch to a chelated TS.
O CCH 3 C H
4 9
2
2 eq C H MgBr CH
C H CH 3 4 9 C H 3
4 9
CH 3 10 mol % CuCN 4 9
OR CH 3
OR
R yield dr(anti:syn)
72 86:4
PhCH 2
CH OCH 2 80 >98:2
3
TBDMS 89 90:10
H 84 7:93
Propargylic acetates, halides, and sulfonates usually react with a double-bond
34
shift to give allenes. Some direct substitution product can be formed as well. A high
ratio of allenic product is usually found with CH Cu-LiBr-MgBrI, which is prepared
3
by addition of methylmagnesium bromide to a 1:1 LiBr-CuI mixture. 35
O 2 CCH 3 H
C CCHC H + CH Cu LiBr MgBrI C C C
5 11
3
5 11 100%
CH 3 C H
Halogens to carbonyl groups can be successfully coupled using organocopper
reagents. For example, 3,9-dibromocamphor is selectively arylated to the carbonyl.
BrCH 2 CH 3
OCH 3 BrCH 2 CH 3
OCH 3
+ ( CuLi
Br ) 2
CH 3
O CH 3
OCH 3 O 79%
CH O Ref. 36
3
Scheme 8.1 gives several examples of the use of coupling reactions of
organocuprate reagents with halides and acetates. Entries 1 to 3 are examples of the
33
J. L. Belelie and J. M. Chong, J. Org. Chem., 67, 3000 (2002).
34 P. Rona and P. Crabbe, J. Am. Chem. Soc., 90, 4733 (1968); R. A. Amos and J. A. Katzenellenbogen,
J. Org. Chem., 43, 555 (1978); D. J. Pasto, S.-K. Chou, E. Fritzen, R. H. Shults, A. Waterhouse, and
G. F. Hennion, J. Org. Chem., 43, 1389 (1978).
35 T. L. Macdonald, D. R. Reagan, and R. S. Brinkmeyer, J. Org. Chem., 45, 4740 (1980).
36
V. Vaillancourt and F. F. Albizatti, J. Org. Chem., 51, 3627 (1992).