Page 708 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 708
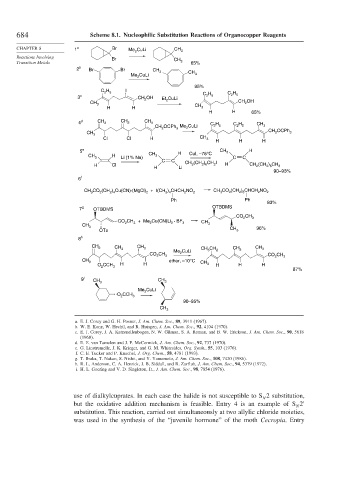
684 Scheme 8.1. Nucleophilic Substitution Reactions of Organocopper Reagents
CHAPTER 8 1 a Br Me 2 CuLi CH 3
Reactions Involving Br CH
Transition Metals 3 65%
2 b Br Br CH
3 CH
Me CuLi 3
2
95%
C H 5 I C H
2
2
3 c CH OH Et CuLi C H 5 2 5
2
CH 3 2 CH OH
2
H H CH 3
H H 65%
4 d CH 2 CH 2 CH 3 C H C H CH
CH OCPh 3 Me 2 CuLi 2 5 2 5 3
2
CH CH 2 OCPh 3
3
Cl Cl H CH 3
H H H
5 e H CH 3 H
H CH 3 CuI, –78°C
CH 3 C C
Li (1% Na)
C C CH (CH ) CH I
H Cl 3 2 6 2 H CH 2 (CH 2 ) 6 CH 3
H Li
90–93%
6 f
CO (CH ) Cu(CN)·(MgCl) + I(CH ) CHCH NO CH CO (CH ) CHCH NO
CH 3 2 2 4 2 2 4 2 2 3 2 2 8 2 2
Ph Ph
83%
7 g OTBDMS OTBDMS
CO CH
2 3
CH + Me Cu(CN)Li · BF
CO 2 3 2 2 3 CH
CH 3 3
OTs CH 3 96%
8 h
CH CH CH
2 3 CH 3 CuLi CH CH 2 CH 3 3
3
CO CH Me 2 CO CH
2 3 2 3
CH 3 ether, –10°C CH
O CCH H H 3 H H H
2 3
87%
9 i
CH 3 CH 3
CuLi
Me 2
O CCH 3
2
90–95%
CH 3
a. E. J. Corey and G. H. Posner, J. Am. Chem. Soc., 89, 3911 (1967).
b. W. E. Konz, W. Hechtl, and R. Huisgen, J. Am. Chem. Soc., 92, 4104 (1970).
c. E. J. Corey, J. A. Katzenellenbogen, N. W. Gilman, S. A. Roman, and B. W. Erickson, J. Am. Chem. Soc., 90, 5618
(1968).
d. E. E. van Tamelen and J. P. McCormick, J. Am. Chem. Soc., 92, 737 (1970).
e. G. Linstrumelle, J. K. Krieger, and G. M. Whitesides, Org. Synth., 55, 103 (1976).
f. C. E. Tucker and P. Knochel, J. Org. Chem., 58, 4781 (1993).
g. T. Ibuka, T. Nakao, S. Nishii, and Y. Yamamoto, J. Am. Chem. Soc., 108, 7420 (1986).
h. R. L. Anderson, C. A. Henrick, J. B. Siddall, and R. Zurfluh, J. Am. Chem. Soc., 94, 5379 (1972).
i. H. L. Goering and V. D. Singleton, Jr., J. Am. Chem. Soc., 98, 7854 (1976).
use of dialkylcuprates. In each case the halide is not susceptible to S 2 substitution,
N
but the oxidative addition mechanism is feasible. Entry 4 is an example of S 2
N
substitution. This reaction, carried out simultaneously at two allylic chloride moieties,
was used in the synthesis of the “juvenile hormone” of the moth Cecropia. Entry