Page 710 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 710
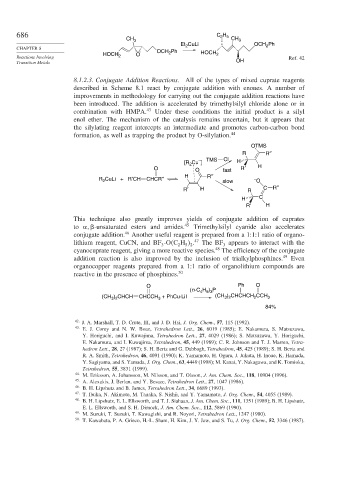
686 C H
2 5
CH 3 CH 3
Et 2 CuLi OCH Ph
2
CHAPTER 8
2
HOCH O OCH Ph HOCH 2
Reactions Involving 2 Ref. 42
Transition Metals OH
8.1.2.3. Conjugate Addition Reactions. All of the types of mixed cuprate reagents
described in Scheme 8.1 react by conjugate addition with enones. A number of
improvements in methodology for carrying out the conjugate addition reactions have
been introduced. The addition is accelerated by trimethylsilyl chloride alone or in
combination with HMPA. 43 Under these conditions the initial product is a silyl
enol ether. The mechanism of the catalysis remains uncertain, but it appears that
the silylating reagent intercepts an intermediate and promotes carbon-carbon bond
formation, as well as trapping the product by O-silylation. 44
OTMS
R R″
– TMS Cl H
[R Cu ]
2
O O fast R′ H
R CuLi + R′CH CHCR″ H R″ slow – O
2
R′ H R C R″
H C
R′ H
This technique also greatly improves yields of conjugate addition of cuprates
to -unsaturated esters and amides. 45 Trimethylsilyl cyanide also accelerates
conjugate addition. 46 Another useful reagent is prepared from a 1:1:1 ratio of organo-
lithium reagent, CuCN, and BF -O C H . 47 The BF appears to interact with the
3
2
5 2
3
48
cyanocuprate reagent, giving a more reactive species. The efficiency of the conjugate
addition reaction is also improved by the inclusion of trialkylphosphines. 49 Even
organocopper reagents prepared from a 1:1 ratio of organolithium compounds are
reactive in the presence of phosphines. 50
O Ph O
H ) P
(n-C 4 9 3
(CH ) CHCH CHCCH + PhCu·LiI (CH ) CHCHCH CCH 3
2
3 2
3
3 2
84%
42
J. A. Marshall, T. D. Crute, III, and J. D. Hsi, J. Org. Chem., 57, 115 (1992).
43 E. J. Corey and N. W. Boaz, Tetrahedron Lett., 26, 6019 (1985); E. Nakamura, S. Matsuzawa,
Y. Horiguchi, and I. Kuwajima, Tetrahedron Lett., 27, 4029 (1986); S. Matsuzawa, Y. Horiguchi,
E. Nakamura, and I. Kuwajima, Tetrahedron, 45, 449 (1989); C. R. Johnson and T. J. Marren, Tetra-
hedron Lett., 28, 27 (1987); S. H. Bertz and G. Dabbagh, Tetrahedron, 45, 425 (1989); S. H. Bertz and
R. A. Smith, Tetrahedron, 46, 4091 (1990); K. Yamamoto, H. Ogura, J. Jukuta, H. Inoue, K. Hamada,
Y. Sugiyama, and S. Yamada, J. Org. Chem., 63, 4449 (1998); M. Kanai, Y. Nakagawa, and K. Tomioka,
Tetrahedron, 55, 3831 (1999).
44
M. Eriksson, A. Johansson, M. Nilsson, and T. Olsson, J. Am. Chem. Soc., 118, 10904 (1996).
45
A. Alexakis, J. Berlan, and Y. Besace, Tetrahedron Lett., 27, 1047 (1986).
46 B. H. Lipshutz and B. James, Tetrahedron Lett., 34, 6689 (1993).
47
T. Ibuka, N. Akimoto, M. Tanaka, S. Nishii, and Y. Yamamoto, J. Org. Chem., 54, 4055 (1989).
48 B. H. Lipshutz, E. L. Ellsworth, and T. J. Siahaan, J. Am. Chem. Soc., 111, 1351 (1989); B. H. Lipshutz,
E. L. Ellsworth, and S. H. Dimock, J. Am. Chem. Soc., 112, 5869 (1990).
49 M. Suzuki, T. Suzuki, T. Kawagishi, and R. Noyori, Tetrahedron Lett., 1247 (1980).
50
T. Kawabata, P. A. Grieco, H.-L. Sham, H. Kim, J. Y. Jaw, and S. Tu, J. Org. Chem., 52, 3346 (1987).