Page 796 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 796
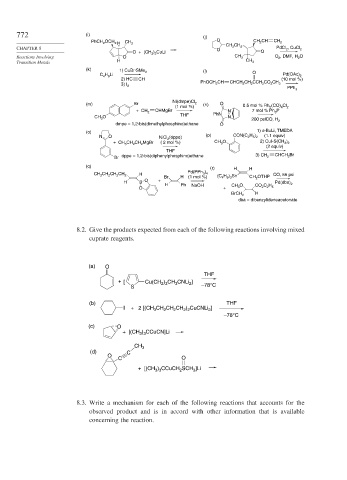
772 (i) (j)
O CH 2 CH
PhCH 2 OCH 2 H CH 3 CH 2
CH 2 CH 2
CHAPTER 8 O PdCl 2 , CuCl 2
O + (CH 3 ) 2 CuLi O
Reactions Involving O CH 3 O 2 , DMF, H 2 O
Transition Metals H CH 3
(k) .
1) CuBr SMe 2 (l) O
C 4 H 9 Li Pd(OAc) 2
2) HC CH (10 mol %)
PhOCH 2 CH CHCH 2 CH 2 CCH 2 CO 2 CH 3
3) I 2
PPh 3
(m) Br Ni(dmpe)Cl 2 (n) O
(1 mol %) 0.5 mol % Rh 2 (CO) 4 Cl 2
CHMgBr 7 mol % Ph 3 P
+ CH 2 N
THF PhN
CH 3 O N
200 pslCO, H 2
dmpe = 1,2-bis(dimethylphosphino)ethane O
(o) 1) s-BuLi, TMEDA
N O NiCl 2 (dppe) (p) CON(C 2 H 5 ) 2 (1.1 equiv)
+ CH 3 CH 2 CH 2 MgBr ( 2 mol %) CH 3 O 2) CuI-S(CH 3 ) 2
(2 equiv)
THF
dppe = 1,2-bis(diphenylphosphino)ethane 3) CH 2 CHCH 2 Br
Br
(q)
(r) H H
H Pd(PPh 3 ) 4 CO, 55 psi
CH 3 CH 2 CH 2 CH 2 (C 4 H 9 ) 3 Sn
Br H (1 mol %) CH 2 OTHP
O +
H B
H Ph NaOH CH 3 O Pd(dba) 2
O + CO 2 C 2 H 5
H
BrCH 2
dba = dibenzylideneacetonate
8.2. Give the products expected from each of the following reactions involving mixed
cuprate reagents.
(a) O
THF
) CH CNLi ]
+ [ Cu(CH 2 3 3 2
S –78°C
(b) THF
I + 2 [(CH CH CH CH ) CuCNLi ]
3
2 2
2
2
2
–78°C
(c) O
+ [(CH ) CCuCN]Li
3 3
CH 3
(d) C
O
C O
+ [(CH ) CCuCH SCH ]Li
3 3
2
3
8.3. Write a mechanism for each of the following reactions that accounts for the
observed product and is in accord with other information that is available
concerning the reaction.