Page 794 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 794
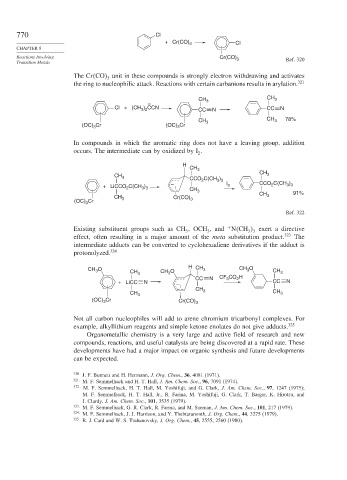
770 Cl
+ Cr(CO) 6 Cl
CHAPTER 8
Reactions Involving Cr(CO) 3 Ref. 320
Transition Metals
The Cr CO unit in these compounds is strongly electron withdrawing and activates
3
the ring to nucleophilic attack. Reactions with certain carbanions results in arylation. 321
CH 3 CH 3
–
Cl + (CH ) CCN CC N CC N
3 2
CH 3 CH 3 78%
Cr (OC) Cr
(OC) 3 3
In compounds in which the aromatic ring does not have a leaving group, addition
occurs. The intermediate can by oxidized by I .
2
H
CH 3
CH 3 CCO C(CH ) CH 3
3 3
2
2
3 3
C(CH ) – I 2 CCO C(CH )
+ LiCCO 2 3 3 CH 3
CH Cr(CO) CH 3 91%
(OC) Cr 3 3
3
Ref. 322
Existing substituent groups such as CH OCH , and + N CH exert a directive
3 3 3 3
effect, often resulting in a major amount of the meta substitution product. 323 The
intermediate adducts can be converted to cyclohexadiene derivatives if the adduct is
protonolyzed. 324
H CH
CH O CH 3 CH O 3 CH 3 O CH 3
3
3
CC N CF CO H
2
3
+ LiCC N – CC N
CH
CH 3 3 CH 3
(OC) Cr Cr(CO) 3
3
Not all carbon nucleophiles will add to arene chromium tricarbonyl complexes. For
example, alkyllithium reagents and simple ketone enolates do not give adducts. 325
Organometallic chemistry is a very large and active field of research and new
compounds, reactions, and useful catalysts are being discovered at a rapid rate. These
developments have had a major impact on organic synthesis and future developments
can be expected.
320
J. F. Bunnett and H. Hermann, J. Org. Chem., 36, 4081 (1971).
321 M. F. Semmelhack and H. T. Hall, J. Am. Chem. Soc., 96, 7091 (1974).
322
M. F. Semmelhack, H. T. Hall, M. Yoshifuji, and G. Clark, J. Am. Chem. Soc., 97, 1247 (1975);
M. F. Semmelhack, H. T. Hall, Jr., R. Farina, M. Yoshifuji, G. Clark, T. Bargar, K. Hirotsu, and
J. Clardy, J. Am. Chem. Soc., 101, 3535 (1979).
323 M. F. Semmelhack, G. R. Clark, R. Farina, and M. Saeman, J. Am. Chem. Soc., 101, 217 (1979).
324 M. F. Semmelhack, J. J. Harrison, and Y. Thebtaranonth, J. Org. Chem., 44, 3275 (1979).
325
R. J. Card and W. S. Trahanovsky, J. Org. Chem., 45, 2555, 2560 (1980).